Table of Contents
Introduction to Chromatography
The separation of mixtures through distribution between two or more immiscible phases is known as chromatography. Chromatography is the most commonly used separation process in chemical laboratories for analysis, isolation, and purification. It is also widely utilized throughout the chemical processing industry, both on a small and large scale.
We apply the mixture that needs to be separated to a stationary phase (solid or liquid), and a pure solvent such as water or any gas is permitted to travel slowly over the stationary component, carrying the components separately according to their solubility in the pure solvent.
Chromatography Definition
The name “chromatography” comes from the Greek terms chroma, meaning “color,” along with graphein, which translates as “to write.”
Principle of Chromatography
Chromatography is a method of separation in which the analyte is mixed with a liquid or gaseous mobile phase that is pushed through a stationary phase. One phase is usually hydrophilic, whereas another is lipophilic.
These two phases interact differently in relation to analyte components. Depending on their polarity, they spend either more or less time within the stationary phase and are thus retarded to varied degrees. As a result, the various components in the sample are separated.
Each sample component elutes from the stationary phase at a different time, which is referred to as the retention time. The components’ signals are captured and displayed in the form of a chromatogram as they travel past the detector.
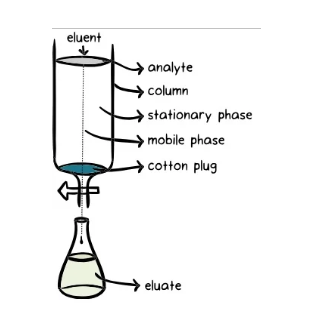
Chromatography Diagram
The chromatography diagram can be shown as follows:
Types of Chromatography
Chromatography techniques can be divided into several types based on the physical state of the mobile and stationary phases. The following are the various types of Chromatography techniques:
- Adsorption Chromatography
- Thin Layer Chromatography
- High Performance Liquid Chromatography
- Partition Chromatography
- Filtration using Gel Liquid chromatography
- Gas Chromatography
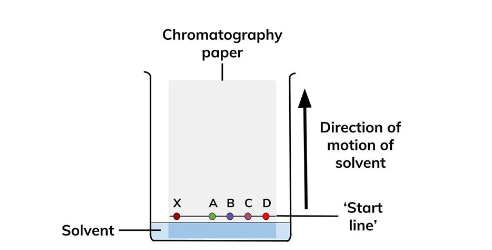
Paper Chromatography
Paper chromatography (PC) is a sort of planar chromatography that includes using specialized paper to conduct chromatography processes.
Because of its usefulness in the isolation, identification, and quantitative determination of organic and inorganic substances, Paper chromatography is regarded as the simplest and most commonly used of the chromatographic procedures.
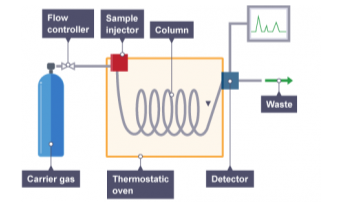
Gas Chromatography
The mobile phase in gas chromatography is a gas, and the components are separated as vapors, which distinguishes it from other types of chromatography.
Gas chromatography is therefore employed in the gas phase to separate and detect tiny molecular weight molecules.
The sample is vaporized in the injection port and is either a gas or a liquid.
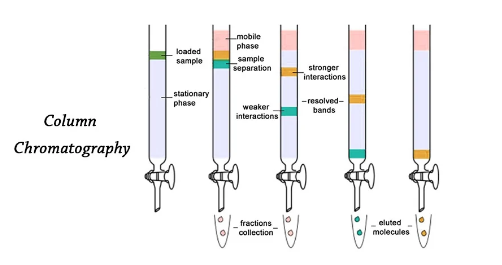
Column Chromatography
Column chromatography is a technique that involves the substances to be separated being introduced onto the top of an adsorbent-packed column, transported through the column at varying rates depending on the affinity of each substance for both the adsorbent and the solvent or just solvent mixture, and frequently gathered in solution as they pass through the column at different periods.
Also Check For:
Thin layer Chromatography
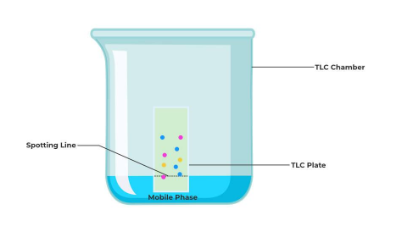
Thin layer chromatography (TLC) uses a glass plate coated with a very thin layer of adsorbent, such as silica gel or alumina, to separate a mixture of compounds into their constituents, as illustrated in the image below.
HPLC Chromatography
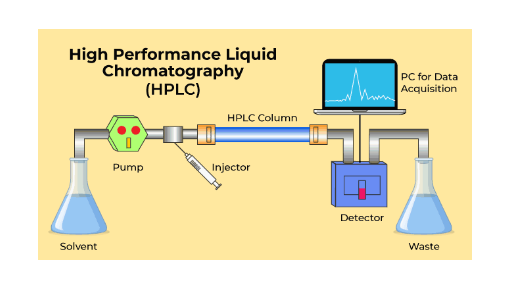
Some of the most important components of chromatographs are columns dedicated to molecular separation and high-performance pumps that deliver solvent at a constant flow rate. This is what is called as HPLC chromatography.
Application of Chromatography
- The application of chromatography are mentioned below:
- A method of separating the colors of a dye.
- Chromatography in the Food Industry
- The Use of Chromatography in the Chemical Industry
- The Use of Chromatography in the Pharmaceutical Industry
Frequently Asked Questions (FAQs) on Chromatography
What is chromatography?
Chromatography is the separation of components in a mixture.
What are the types of chromatography principle?
In laboratories, chromatography is used for substance separation, purification, and testing. Adsorption Chromatography and Partition Chromatography are the two primary forms of chromatography. Column chromatography, thin layer chromatography, and paper chromatography are the three major chromatography techniques.
What's the Rf value?
The retention factor, often known as the Rf value, is a measure of the location of a component in a chromatographic separation. It is computed by dividing the component's distance traveled by the solvent's distance traveled.
What is stationary phase?
In chromatography, a stationary phase is one that does not move with the sample, whereas a mobile phase moves with the sample. As an example: In paper chromatography, the paper strip serves as the stationary phase, and the solvent serves as the mobile phase.









