Table of Contents
Introduction to SN1 Reaction
In organic chemistry, substitution reactions play a vital role in the transformation of one functional group into another. Among these reactions, the SN1 reaction and SN2 reaction are fundamental and extensively studied.
Both SN1 (Substitution Nucleophilic Unimolecular) and SN2 (Substitution Nucleophilic Bimolecular) reactions involve the replacement of a leaving group with a nucleophile. SN1 Reaction Class 12 is an essential topic covered in class 12 chemistry curriculum. Understanding the mechanism and characteristics of SN1 reactions is crucial for students’ knowledge of organic chemistry.
In this article, we will delve into the mechanism, examples, stereochemistry, and a detailed comparison between the SN1 reaction and SN2 reaction.
SN1 Definition
SN1 stands for Substitution Nucleophilic Unimolecular, where the rate-determining step involves only one reactant. The SN1 reaction is a type of nucleophilic addition substitution reaction.
It happens with both carbonyl and benzene molecules. In other words, electrophiles are attacked by an electron-rich nucleophile in this process. These are also referred to as SN1 reactions or first order reactions.
SN1 Reaction Steps
The SN1 reaction steps are as follows:
- The departure of the leaving group
- The nucleophile’s attack on the carbocation
- Deprotonation to produce neutral product
The SN1 reaction mechanism is a step-by-step approach in which the carbocation is created first by removing the leaving group.
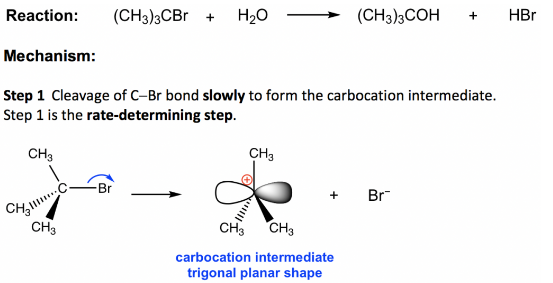
The nucleophile then attacks the carbocation.

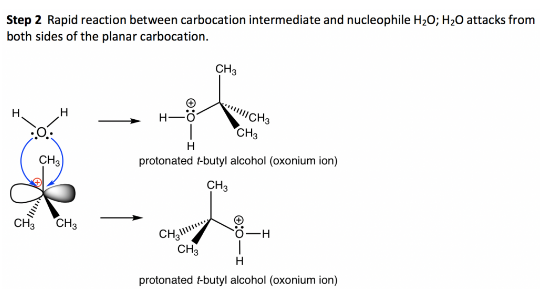
Finally, the protonated nucleophile is deprotonated to yield the desired product. The electrophilicity of the leaving group determines the pace of this reaction, which is unaffected by the nucleophile.
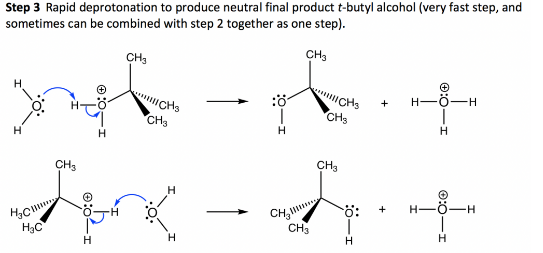
The reaction rate depends on the concentration of the substrate and not the nucleophile.
SN1 Reaction Reactivity Order
The SN1 Reaction Reactivity Order depends on the stability of the carbocation intermediate. More substituted carbocations are more stable, leading to higher reactivity. The SN1 Reaction Reactivity Order for the carbocations is as follows:

SN2 Definition
‘SN2’ stands for Substitution Nucleophilic Bimolecular Reaction, where one bond is broken and another is created at the same time.
Mechanism of SN2 Reaction
In SN2 reactions, the nucleophile attacks the carbon center simultaneously as the leaving group departs. The nucleophile approaches from the other side of the leaving group, causing a configuration inversion. The SN2 reaction is as follows:
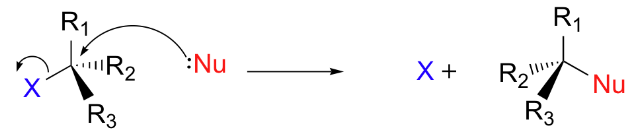
The mechanism of SN2 Reaction is mentioned below:
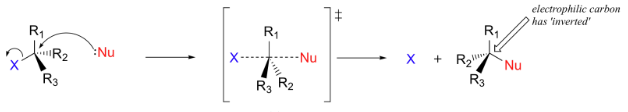
SN1 Reaction vs SN2 Reaction
SN1 and SN2 reactions have distinct characteristics.
SN1 reactions prefer polar protic solvents, proceed through carbocation intermediates, and are unimolecular in rate-determining steps.
On the other hand, SN2 reactions favor polar aprotic solvents, proceed through concerted mechanisms, and are bimolecular in rate-determining steps.
Both the SN1 Reaction and SN2 Reaction are nucleophilic substitution reactions, but they differ in their reaction conditions and mechanisms.
SN1 and SN2 Reaction Mechanism
The SN1 reaction and SN2 reaction have different mechanisms as mentioned above.
- The SN1 reaction proceeds in two steps, starting with the formation of a carbocation intermediate. In the first step, the leaving group departs, generating a positively charged carbon atom. In the second step, a nucleophile attacks the carbocation to complete the substitution.
- In contrast, SN2 reactions occur in a single concerted step where the nucleophile directly displaces the leaving group. This is the major difference between the SN1 and SN2 reaction mechanisms.
Stereochemistry of SN1 and SN2 reaction
The stereochemistry of SN1 reactions involves the racemization of chiral centers, as the carbocation intermediate can be attacked from either side. In contrast, SN2 reactions result in the inversion of stereochemistry.
SN1 and SN2 Reactions Examples
SN1 and SN2 reactions examples exist in organic chemistry. A classic SN1 example is the solvolysis of tert-butyl bromide in ethanol, leading to the formation of tert-butyl alcohol. On the other hand, an example of an SN2 reaction is the nucleophilic substitution of methyl chloride with a hydroxide ion to produce methanol.
SN1 and SN2 Reaction Mechanism PDF
Students often refer to PDF documents to gain a comprehensive understanding of SN1 and SN2 reaction mechanisms. These resources provide detailed, step-by-step explanations of the reactions.
Frequently Asked Questions on SN1 Reaction
What is an example of an SN1 nucleophile?
Typical SN1 reactions occur when the solvent acts as the nucleophile. Examples of nucleophiles include H
What is the difference between SN1 and SN2 reactions?
The main difference between SN1 and SN2 reactions lies in their mechanisms and stereochemistry. SN1 reactions proceed through a carbocation intermediate, while SN2 reactions occur in a single concerted step without any intermediate formation. SN2 has a complete configuration inversion. If the alkyl halide is optically active in the SN1 reaction, the result is a racemic mixture. Nucleophile assaults occur from both the backend and the front side of the SN1 reaction.
What are SN2 reactions used for?
SN2 reactions are used in organic synthesis to introduce various functional groups into organic compounds. They are particularly useful for the preparation of nucleoside analogs, pharmaceuticals, and other important organic molecules.
Why is it called SN1 and SN2?
SN1 and SN2 are called based on their reaction mechanisms. SN1 stands for Substitution Nucleophilic Unimolecular, while SN2 stands for Substitution Nucleophilic Bimolecular, reflecting their respective stepwise and concerted mechanisms.








