Table of Contents
Combustion and Flame Class 8 Extra Questions Science Chapter 6
Combustion and Flame Class 8 Extra Questions Very Short Answer Questions
Question 1.
Name the most common fuel used in homes.
Answer:
Liquefied Petroleum Gas. (LPG)
Question 2.
Name the most common fire extinguisher.
Answer:
Water
Question 3.
What are the states in which a fuel may exist?
Answer:
A fuql may exist in solid, liquid or gaseous state.
Question 4.
Name any two combustible substances.
Answer:
Charcoal, wood
Question 5.
Does magnesium produce heat and light during its combustion?
Answer:
Yes, it does.
Question 6.
What acts as a fuel for our body?
Answer:
Food
Question 7.
Give two examples of non-combustible substances. .
Answer:
Water, sand
Question 8.
How are heat and light produced in the sun?
Answer:
In the sun, heat and light are produced by nuclear reactions.
Question 9.
Where were matchsticks first used?
Answer:
Egypt
Question 10.
What are the three essential requirements for combustion?
Answer:
Fuel, air (to supply oxygen) and heat (to raise the temperature of the fuel beyond the ignition temperature).
Question 11.
What is the ignition temperature of phosphorus?
Answer:
25°C
Question 12.
What is the colour of an LPG flame?
Answer:
Blue
Question 13.
What type of process is combustion?
Answer:
A chemical process
Question 14.
Name an ideal fuel.
Answer:
Compressed Natural Gas (CNG)
Question 15.
What is the composition of the head of a matchstick?
Answer:
Antimony trisulphide and potassium chlorate.
Question 16.
Which part of a flame does a goldsmith blow for melting gold and silver?
Answer:
The goldsmith blows the outermost zone of a flame for melting gold and silver.
Question 17.
What is the unit for expressing the calorific value of a fuel?
Answer:
Kilojoules per kilogram (kJ/kg)
Question 18.
Comparing the calorific values of coal and petrol, state which fuel is better.
Answer:
The calorific value of coal is about 25,000 – 33,000 kJ/kg, whereas that of petrol is 45,000 kJ/kg. Hence, petrol is a better fuel.
Question 19.
What is deforestation?
Answer:
The cutting down of trees on a large scale is termed as deforestation.
Question 20.
Give any two examples of carbon fuels.
Answer:
Coal, petroleum
Combustion and Flame Class 8 Extra Questions Short Answer Questions
Question 1.
What does magnesium burn to form?
Answer:
Magnesium burns to form magnesium oxide and produces heat and light.
Question 2.
What does coal produce during its combustion?
Answer:
Coal produces carbon dioxide, heat and light during its combustion.
Question 3.
What is combustion?
Answer:
Combustion is a chemical process in which a substance reacts with oxygen to give off heat.
Question 4.
Define ignition temperature of a fuel.
Answer:
The lowest temperature at which a fuel catches fire is called its ignition temperature.
Question 5.
How does a matchstick catch fire?
Answer:
By rubbing a matchstick against a rough surface (friction), it attains its ignition temperature and thus catches fire.
Question 6.
Why is sodium kept immersed in kerosene?
Answer:
Sodium has very low ignition temperature, i.e., it catches fire on coming in contact with air, so it is kept in kerosene.
Question 7.
What are combustible and non-combustible substances?
Answer:
Substances which undergo combustion are said to be combustible, whereas non-combustible substances are those which don’t burn.
Question 8.
What are inflammable substances? Give examples.
Answer:
The substances which have very low ignition temperature and can easily catch fire with a flame are called inflammable substances; e.g., LPG, petrol, alcohol, etc.
Question 9.
What is rapid combustion?
Answer:
When a substance burns instantly and produces a huge amount of heat and light, the combustion is called rapid combustion; e.g., the instant burning of LPG in a gas stove.
Question 10.
Define spontaneous combustion.
Answer:
A type of combustion in which the substance suddenly catches fire without the supply of heat or friction externally is called spontaneous combustion; e.g., forest fires.
Question 11.
Define explosion.
Answer:
A type of combustion during which a huge amount of heat and light is evolved with a boom, along with the production of gas, is known as explosion; e.g., the exploding of fireworks, i.e., crackers, etc.
Question 12.
What is flame?
Answer:
Flame is a region where the burning or combustion of gaseous substances take place.
Question 13.
Define fuel.
Answer:
Those substances which provide energy on burning are called ‘fuels’; e.g., coal, petroleum, LPG, etc.
Question 14.
Give two examples each of solid fuels, liquid fuels and gaseous fuels.
Answer:
Solid fuels – Wood, cow dung, etc.
Liquid fuels – Kerosene, petrol, etc.
Gaseous fuels – Hydrogen, methane, etc.
Question 15.
Give two examples of fuels that are used to generate electricity.
Answer:
Two examples of fuels that are used to generate electricity are petrochemicals and coal.
Question 16.
Define calorific value.
Answer:
The amount of heat produced on burning one kilogram of fuel completely is called its calorific value.
Question 17.
60 kg of fuel was completely burnt for an experiment. The amount of heat energy was found to be 1,80,000 kJ. Calculate the calorific value of the fuel.
Answer:
Amount of fuel burnt = 60 kg
Amount of heat produced = 1,80,000 kJ
Calorific value of the fuel = \(\frac{\text { Heat produced }}{\text { Amount of fuel }}\)
= \(\frac{1,80,000}{60}\) = 3,000 kJ/kg
∴ Calorific value of the fuel is 3,000 kJ/kg.
Question 18.
Define dark zone of a flame.
Answer:
The innermost zone of a flame around the wick is called its dark zone. It is the least hottest zone com¬paratively to other.
Question 19.
Name the colours of the flames of following substances:
| Barium, arsenic, sodium, magnesium. |
Answer:
| Name of the substance | Colour of its flame |
| Barium | Pale/Apple green |
| Arsenic | Blue |
| Sodium | Yellow |
| Magnesium | White |
Question 20.
Mention any three characteristics of a good fuel.
Answer:
Any three characteristics of a good fuel are following:
- It has high calorific value.
- It is very easy to transport.
- It is cheap, affordable and economic.
Question 21.
What is global warming?
Answer:
An increase in the average temperature of the earth’s atmosphere, especially a sustained increase that causes climatic changes, is termed as ‘global warming’.
Combustion and Flame Class 8 Extra Questions Long Answer Questions
Question 1.
Why isn’t hydrogen gas used as a domestic or industrial fuel, although it has a very high calorific value? State three reasons for the answer.
Answer:
Although hydrogen gas has a very high calorific value, it is not used as a domestic or industrial fuel due to the following reasons:
- It is expensive.
- It burns with an explosion.
- It is extremely inflammable, so it is risky to store and transport hydrogen.
Question 2.
Explain how water gets boiled in paper cup without burning it.
Answer:
When we heat the paper cup containing water, the heat given to it is rapidly transferred to water from the paper cup. The temperature of water goes on increasing until it attains its boiling point, and starts boiling. As, during this process, the heat is continuously being transferred to water; the paper cup does not attain its ignition temperature. Hence, it does not burn.
Question 3.
Why does a piece of paper burn with yellow flame? Give a reason.
Answer:
The inadequate supply of oxygen during the combustion of the piece of paper produces solid carbon particles that rise up in the flame. They become hot and glow to give off yellowish light. This makes the piece of paper burn with a yellowish flame.
Question 4.
It is observed at petrol pumps and airports, that hydrocarbon fire extinguishers are used, instead of soda-acid fire extinguisher. Give reasons why.
Answer:
At petrol pumps and airports, there is more probability of fire break out due to oil. In such situations, soda-acid fire extinguisher does not work as it contains water or uses water to take off fire by cooling down the place. Water being heavier than oil sinks to bottom and hence, fire does not get controlled. In such a case, hydrocarbon fire extinguisher is very useful, as it contains turkey red oil, which causes the foaming of carbon dioxide gas under pressure. The foam covers the surface of the burning substances and dispels the supply of air to control fire.
Question 5.
Explain complete combustion.
Answer:
This type of combustion involves complete burning of the combustible substance. No residue is left behind. Ash or smoke is not given off during or after this type of combustion. Mostly gases, such as hy¬drocarbons go through this form of combustion. On combustion, hydrocarbon produces carbon dioxide, water and heat.
Combustion and Flame Class 8 Extra Questions Higher Order Thinking Skills
Question 1.
There are three factors which supports burning. Fire extinguisher affects which factor?
Answer:
Fire extinguishers affect the supply of air.
Question 2.
Although wood has a very high calorific value, we still discourage its use as a fuel. Explain.
Answer:
Wood pollutes air very much and using wood as a fuel would also lead to deforestation on a large scale.
So we still discourage wood as a fuel.
Question 3.
Write in brief about the reasons of forest fires.
Answer:
Reasons of forest fires are:
- lightning if strikes forest trees or areas may lead to forest fires.
- human-caused fires.
- during extreme heat of summer, at some places dry grass catches fire. From this grass, very soon, it spreads to the whole forest.
- sparks from rock falls in a mountainous region can also be the reason of forest fires.
- volcanic eruption can also cause forest fires.
Question 4.
Can the process of cellular respiration be called combustion? Why?
Answer:
Yes. Cellular respiration is a set of metabolic reactions and processes that breaks large molecules into smaller one with the release of heat. So, this is a slow combustion reaction.
Question 5.
If you hold a piece of iron wire with a pair of tongs inside a candle flame or a Bunsen burner flame, what will you observe? Will it produce a flame?
Answer:
We will observe that the iron wire will become red hot and start glowing but it will not produce a flame as it is non flammable object.
Combustion and Flame Class 8 Extra Questions Value-Based Questions
Question 1.
Sourav was heating oil to fry potato chips. The cooking oil all of a sudden caught fire. He took water to pour on the fire to extinguish it. But meanwhile his mother came and switched off the gas and covered the wok completely with a plate.
- Do you think pouring water to the burning oil would have worked? Why?
- Do you think what Sourav’s mother had done is right? Why?
- Can you suggest other ways in which we can stop fire due to burning oil?
- What values of Sourav and Sourav’s mother are shown here?
Answer:
- No. As oil being lighter than water will come up and spill all around resulting in a major accident.
- Sourav’s mother had done right because she cut off the heat and oxygen supply from the oil. The fire will ultimately extinguish if it doesn’t get the required supply of air.
- (c)
- By using excess of baking soda.
- By using fire extinguisher.
- By putting a completely wet towel on the pan to cut off the air supply.
- Sourav is immature in handling such situation but his mother is mature, wise with scientific temperament.
Question 2.
During a class discussion on ‘fuels for household’ Sarita suggested petrol. But her teacher said it is not a safe fuel for household activity and asked her to sit. Sarita wondered why petrol can’t be used as a fuel for household activity though its calorific value is high.
- Why petrol is not a safe fuel for household activity?
- What fuels do we use in our houses?
- What value of Sarita is shown here?
Answer:
- Petrol vapourises easily so it can lead to rapid combustion. Hence it is not safe as household fuel.
- We use LPG, kerosene and wax.
- Sarita has knowledge of fuel but she is little bit confused.
Activities and Projects
Question 1.
Survey the availability of various fuels in your locality. Find out their cost per kg and pre¬pare a tabular chart showing how many kJ of various fuels you can get for every rupee.
Answer:
Do it yourself.
Question 2.
Find out the number, type and location of fire extinguishers available in your school, nearby shops and factories. Write a brief report about the preparedness of these establishments to fight fire.
Answer:
Do it yourself.
Question 3.
Survey 100 houses in your area. Find the percentage of households using LPG, kerosene, wood and cattle dung as fuel.
Answer:

Question 4.
Talk to people who use LPG at home. Find out what precautions they take in using LPG.
Answer:
Some precautions taken by them are following:
- Cleaning of gas burner regularly and properly.
- Changing the delivery pipe regularly.
- Making use of strong delivery pipes.
- Check-up of related appliances at regular intervals.
Question 5.
Make a model of a fire extinguisher. Place a short candle and a slightly taller candle in a small dish filled with baking soda. Place the dish at the bottom of a large bowl. Light both the candles. Then pour vinegar into the dish of baking soda. Take care. Do not pour vinegar on the candles. Observe the foaming reaction. What happens to the candles? Why? In what order?
For more information, visit:
- www.newton.dep.anl.gov/askasci/chem03/chem03767.htm
- http://en.wikipedia.org/wiki/combustion
- http://wwwchem.csustan.edu/consumer/fuels/heats%20.htm
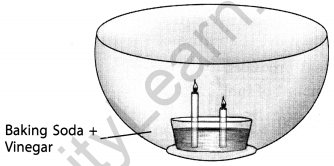
Answer:
It is observed that the candles go off. The shorter candle blows off first, and then the longer one. It hap¬pens because of the production of carbon dioxide gas, that reaches the shorter candle first, and then the longer candle.
I. Multiple Choice Questions (MCQs)
Choose the correct option.
Question 1.
Which of the following fuels is used for running automobiles?
(a) CNG
(b) Petrol
(c) Both (a) and (b)
(d) Wood
Question 2.
Magnesium burns to form
(a) calcium carbonate
(b) magnesium oxide
(c) calcium oxide
(d) magnesium sulphate
Question 3.
Coal burns to produce
(a) calcium bicarbonate
(b) magnesium
(c) carbon dioxide
(d) oxygen
Question 4.
Name the chemical process in which a substance reacts with oxygen to give out heat.
(a) Reaction
(b) Junction
(c) Combustion
(d) All of these
Question 5.
The substance that undergoes combustion is said to be
(a) burning
(b) flame
(c) charcoal
(d) combustible
Question 6.
Combustible substances are also known as
(a) inflammable
(b) flaming
(c) illuminous
(d) non-flammable
Question 7.
Which of the following is a combustible?
(a) Stone piece
(b) Wood
(c) Glass
(d) None of these
Question 8.
In the sun, light and heat are produced by
(a) chemical reactions
(b) nuclear reactions
(c) burning reactions
(d) bunsen burner
Question 9.
Lowest temperature at which a substance catches fire is known as
(a) lowest temperature
(b) burning temperature
(c) ignition temperature
(d) flaming temperature
Question 10.
Long, long ago, which of the following trees was used to produce matchsticks?
(a) Mango
(b) Deodar
(c) Banyan
(d) Pine
Question 11.
Which chemical is used in the rubbing surface provided for matchsticks?
(a) Sulphur
(b) Gold
(c) Red phosphorus
(d) White phosphorus
Question 12.
Substances which have very low ignition temperature and can catch fire easily are called
(a) flammable substances
(b) inflammable substances
(c) combustible substances
(d) all of these
Question 13.
Which of the following is an example of inflammable substance?
(a) Iron
(b) Glass
(c) LPG
(d) Stone
Question 14.
Which of the following are required essentially for producing fire?
(a) Glass, coal, water
(b) Fuel, coal, straw
(c) Fire, wood, burner
(d) Fuel, air, heat
Question 15.
The most common element used as fire extinguisher is
(a) CO2
(b) oxygen
(c) phosphorous
(d) oxygen
Question 16.
Baking soda constitutes
(a) hydrogen chloride
(b) sodium oxide
(c) sodium bicarbonate
(d) oxygen
Question 17.
LPG means
(a) Liquefied Petroleum Gas
(b) Liquefied Petrol Gas
(c) Liquid Petrol Godown
(d) Liquid Petroleum Gas
Question 18.
Phosphorus burns at
(a) room temperature
(b) 100°C
(c) cool temperature
(d) any temperature
Question 19.
‘Firework’ is an example of
(a) rapid combustion
(b) explosion
(c) spontaneous combustion
(d) slow combustion
Question 20.
The calorific value of‘hydrogen’ is
(a) 50,000 kJ/kg
(b) 55,000 kJ/kg
(c) 1,50,000 kJ/kg
(d) 6,000 kJ/kg
Answer:
1. (c)
2. (b)
3. (c)
4. (c)
5. (d)
6. (a)
7. (b)
8. (b)
9. (c)
10. (d)
11. (c)
12. (d)
13. (c)
14. (d)
15. (a)
16. (c)
17. (a)
18. (a)
19. (b)
20. (c)
II. Fill in the Blanks
Fill in the blanks with suitable word/s.
1. The substance which vapourises during burning gives _________ .
2. A good fuel should have _________ calorific value.
3. An example of slow combustion is _________.
4. _________ are substances that release energy on combustion.
5. The most common supporter of combustion is _________.
6. Magnesium burns to change into _________.
7. Wood is an example of _________ fuel.
8. _________ is an example of liquid fuel.
9. Carbon dioxide is _________ than oxygen.
10. Oxides of _________ and nitrogen causes acid rain.
11. The increase in amount of _________ gas in atmosphere results in global warming.
12. Inflammable substances have very low _________ temperature.
13. The _________ zone of a flame is the hottest.
14. During combustion, generally, _________ and _________ energies are produced.
15. LPG has a calorific value of _________ kJ/kg.
Answer:
1. flame
2. high
3. respiration
4. Fuels
5. oxygen/air
6. magnesium oxide
7. solid
8. Kerosene
9. heavier
10. sulphur
11. carbon dioxide
12. ignition
13. non-luminous
14. heat, light
15. 55,000
III. Match the following
Match the items given in column I suitably with those given in column II.
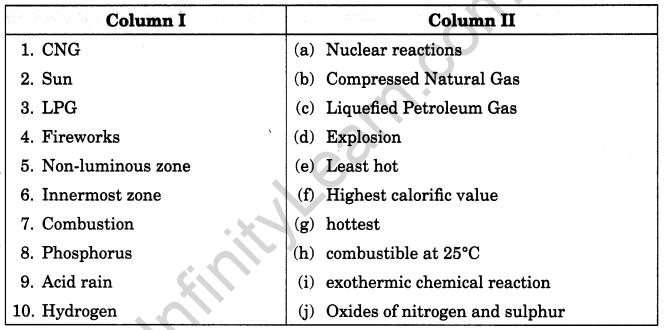
Answer:
1. (b)
2. (a)
3. (c)
4. (d)
5. (g)
6. (e)
7. (i)
8. (h)
9. (j)
10. (f)
IV. True or False
State whether the given statements are true or false.
1. Automobiles run only by using petrol.
2. CNG means ‘Connecting Neutral Gas’.
3. Coal burns with a flame.
4. Magnesium burns to produce magnesium oxide.
5. Combustion is a physical process.
6. The substance that undergoes combustion is said to be combustible.
7. Fuel may be solid, liquid or gas.
8. Wood is combustible.
9. A matchstick only contains white phosphorus.
10. Kerosene is an example of solid fuel.
11. For combustion, a substance must attain its ignition temperature.
12. The ignition temperature of phosphorus is 25°C.
13. LPG is an example of inflammable substances.
14. Water is a bad conductor of electricity.
15. Fuel, water and heat are three essential requirements for combustion.
16. The most common fire extinguisher is water.
17. Oxygen is heavier than C02.
18. The middle zone of a flame has yellow colour.
19. The calorific value of hydrogen is 1,50,000 kJ/kg.
20. Increase in nitrogen gas in atmosphere has led to ‘global warming’.
Answer:
1. False
2. False
3. False
4. True
5. False
6. True
7. True
8. True
9. False
10. False
11. True
12. True
13. True
14. False
15. False
16. True
17. False
18. True
19. True
20. False









