To Study the Reaction Rate of Reaction of Iodide Ions with Hydrogen Peroxide at Different Concentrations of Iodide Ions
Theory
Hydrogen peroxide oxidizes iodide ions to iodine in acidic medium
H2O2 + 2I– + 2H+ —–> 2H20 + I2
The reaction is monitored by adding a known volume of sodium thiosulphate solution and starch solution to the reaction mixture. Iodine liberated at once reacts with sodium
thiosulphate solution and is reduced to iodide ions
I2+ 2S2O32- —fast—> S8O62- + 2I–
When thiosulphate ions are completely consumed, the liberated iodine reacts with starch solution and gives blue colour
I2 + Starch ——-> Blue complex
The time elapsed before the appearance of blue colour, gives an idea about the rate of the reaction.
Apparatus and Chemicals
4 Conical flasks (250 ml), measuring cylinder, burette, pipette (25 ml), stop-watch, 0.1 M KI t solution, 2.5 M H2S04, starch solution. ‘3%’ H202 solution, 0.05 M sodium thiosulphate solution.
Procedure
- Take four 250 ml conical flasks and label them as A, B, C and D.
- Add 10 ml, 20 ml, 40 ml and 60 ml of 0.1 M KI solution to the flasks A, B, C and D respectively.
- Add 10 ml of to each flask.
- Add water to make the volume of solution 100 ml in each flask.
- Add 5 ml starch solution to each flask.
- Add 10 ml of 0.05 M sodium thiosulphate solution to each flask.
- Add 5 ml of 3% hydrogen peroxide solution to flask A with the help of a pipette and start the stop watch immediately. Stir the mixture and watch for the blue colour to appear. Note the time when the blue colour just appears.
- Repeat the step 7 with the solutions in flasks B, C and D.
Observations
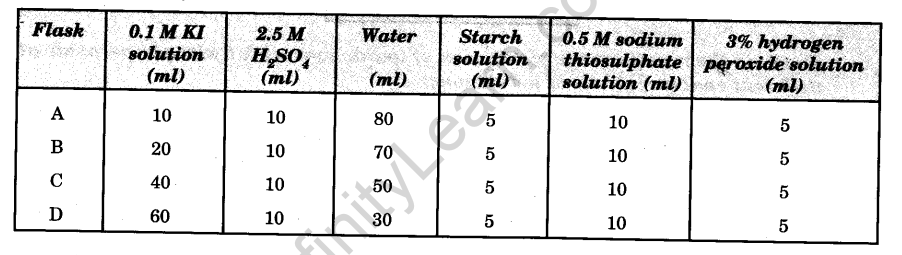
Time required for the blue colour to first appear in :
Flask A — ………..s
Flask B — ……….s
Flask C — ……….s
Flask D — ……….s
Conclusion
The rate of the reaction increases with increase in concentration of iodide ions.
Precautions
- Always use a freshly prepared solution of sodium thiosulphate.
- Concentration of KI solution should be higher than the concentration of sodium thiosulphate solution.
- Use freshly prepared starch solution.





