Table of Contents
Introduction to Bicarbonate Formula
Bicarbonate, also known as hydrogen carbonate, is a chemical compound with the formula HCO3-. It is an anion that consists of one carbon atom, one hydrogen atom, and three oxygen atoms. Bicarbonate is an important species in chemistry and biochemistry, playing various roles in different systems. It is commonly found in nature, including in minerals such as calcite and aragonite. In biological systems, bicarbonate is present in the form of dissolved carbon dioxide (CO2) in the blood, where it helps maintain the acid-base balance and is involved in the transport of carbon dioxide.
Uses of Bicarbonate Formula
- Baking: Bicarbonate, commonly known as baking soda, is widely used as a leavening agent in baking. When combined with an acidic ingredient, such as buttermilk or vinegar, it reacts to produce carbon dioxide gas, causing dough or batter to rise.
- Antacid: Bicarbonate is used as an antacid to relieve heartburn, indigestion, and acid reflux. It helps neutralize excess stomach acid by reacting with the acid to form water and carbon dioxide.
- Medical applications: Bicarbonate is used in medical treatments to manage certain health conditions. For example, intravenous administration of bicarbonate can help correct acid-base imbalances in cases of severe metabolic acidosis.
- Cleaning agent: Bicarbonate is an effective and environmentally friendly cleaning agent. It can be used to scrub and remove stains from various surfaces, including countertops, sinks, and bathroom fixtures.
- Fire extinguisher: Bicarbonate-based fire extinguishers, also known as Class B/C fire extinguishers, are commonly used to put out fires involving flammable liquids and electrical equipment. Bicarbonate reacts with fire by releasing carbon dioxide gas, which displaces oxygen and helps smother the flames.
- pH buffering: Bicarbonate acts as a pH buffer in various applications. It helps maintain stable pH levels in swimming pools, aquariums, and water treatment processes.
- Agriculture: Bicarbonate is sometimes used in agriculture to correct soil alkalinity and adjust pH levels. It can help improve nutrient availability to plants and promote healthy growth.
Structural Formula of Bicarbonate Formula
Bicarbonate is a polyatomic ion consisting of one carbon atom, one hydrogen atom, and three oxygen atoms. It has a negative charge, which is balanced by the presence of a positively charged ion in chemical compounds.
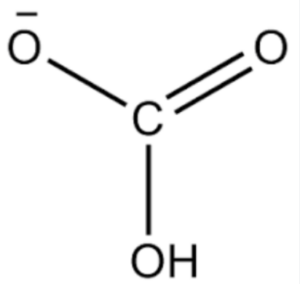
It is important to note that this representation simplifies the structure and does not accurately reflect the actual three-dimensional arrangement of the atoms in space. The bicarbonate ion exists as a planar structure with a trigonal planar geometry around the central carbon atom.
The bicarbonate ion (HCO3-) does not have a specific structural formula because it is an ion, meaning it does not exist as a discrete molecule. However, we can represent the bicarbonate ion using a Lewis dot structure to show the arrangement of atoms and their valence electrons.
In the Lewis dot structure of the bicarbonate ion:
- the carbon atom is represented by the letter C
- the hydrogen atom by H
- the oxygen atoms by O
Physical Properties of Bicarbonate
- State of Matter: Depending on the specific compound, substances containing bicarbonate ions can exist as solids, liquids, or gases. For example, sodium bicarbonate (NaHCO3) is a white crystalline solid at room temperature, while sodium bicarbonate dissolved in water forms a clear aqueous solution.
- Melting and Boiling Points: The melting and boiling points of substances containing bicarbonate ions vary depending on the specific compound. For instance, sodium bicarbonate has a melting point of around 270°C (518°F).
- Solubility: Bicarbonate compounds can exhibit varying degrees of solubility in water and other solvents. For example, sodium bicarbonate is highly soluble in water, while calcium bicarbonate (Ca(HCO3)2) is less soluble.
- pH: Bicarbonate ions are amphiprotic, meaning they can act as both acids and bases. In water, bicarbonate ions can react with hydrogen ions to release carbon dioxide gas, thereby acting as a weak acid. This property contributes to the buffering capacity of bicarbonate compounds.
- Electrical Conductivity: Bicarbonate compounds in an aqueous solution can conduct electricity due to the presence of dissociated ions. The degree of conductivity depends on the concentration of bicarbonate ions and other ions in the solution.
Chemical properties of Bicarbonate
- Amphiprotic Nature: Bicarbonate ions can behave as both acids and bases. In the presence of a strong acid, bicarbonate ions can accept a proton (H+) and act as a base, forming carbonic acid (H2CO3). Conversely, in the presence of a strong base, bicarbonate ions can donate a proton and act as an acid, forming carbonate ions (CO32-).
- Acid-Base Reactions: Bicarbonate ions readily participate in acid-base reactions. For example, when bicarbonate ions react with strong acids, such as hydrochloric acid (HCl), carbon dioxide gas (CO2) is released. This reaction can be observed when baking soda (sodium bicarbonate) reacts with an acid, producing carbon dioxide bubbles.
- Buffering Capacity: Bicarbonate ions play a crucial role in maintaining the pH balance in biological systems. They act as a buffer, helping to regulate and stabilize the pH by accepting or donating protons as needed. This buffering capacity is important in maintaining the pH of blood and other bodily fluids.
- Decomposition: At high temperatures, bicarbonate ions can decompose into water, carbon dioxide, and a metal oxide. For example, sodium bicarbonate (NaHCO3) decomposes when heated, producing water vapor (H2O), carbon dioxide gas (CO2), and sodium carbonate (Na2CO3).
- Solubility: Bicarbonate compounds can exhibit varying solubilities in water and other solvents. For example, sodium bicarbonate (NaHCO3) is highly soluble in water, while calcium bicarbonate (Ca(HCO3)2) is less soluble.
Conclusion
In conclusion, the bicarbonate formula, HCO3-, is a versatile compound with numerous applications. It is widely used in baking as a leavening agent and in antacid medications to neutralize excess stomach acid. Bicarbonate also finds use in medical treatments, cleaning agents, fire extinguishers, pH buffering, and agricultural practices. Its ability to react with acids, release carbon dioxide, and act as a pH buffer makes it valuable in various industries and everyday uses. Bicarbonate plays an important role in maintaining proper chemical balance, promoting safety, and enhancing the effectiveness of various processes.
Solved Examples on Bicarbonate Formula
Example 1: What is the balanced chemical equation for the reaction between sodium bicarbonate (NaHCO3) and hydrochloric acid (HCl)?
Solution:
The balanced chemical equation for the reaction is:
NaHCO3 + HCl → NaCl + H2O + CO2
In this reaction, sodium bicarbonate reacts with hydrochloric acid to form sodium chloride, water, and carbon dioxide.
Example 2: Calculate the molar mass of potassium bicarbonate (KHCO3).
Solution:
To calculate the molar mass of potassium bicarbonate, we sum up the atomic masses of its constituent elements:
- K: 39.10 g/mol
- H: 1.01 g/mol
- C: 12.01 g/mol
- O: 16.00 g/mol
Molar mass of KHCO3 = (39.10) + (1.01) + (12.01) + (3 * 16.00)
= 100.11 g/mol
The molar mass of potassium bicarbonate is 100.11 g/mol.
Frequently Asked Questions on Bicarbonate Formula
Why is HCO3 called bicarbonate?
HCO3 is called bicarbonate as it's a carbonate (CO3) with one extra hydrogen (H) atom, indicating a midway point between carbonic acid (H2CO3) and carbonate ion (CO3^2-).
What is the full name of bicarbonate?
The full name of bicarbonate is Hydrogen Carbonate, representing its composition of hydrogen, carbon, and oxygen.
Why is HCO3 called bicarbonate?
(Repeat) HCO3 is termed bicarbonate as it carries a single hydrogen atom, signifying a stage between carbonic acid and the carbonate ion.
What is sodium bicarbonate formula used for?
Sodium bicarbonate formula (NaHCO3) is primarily used as baking soda in cooking, as an antacid for stomach acid issues, and in fire extinguishers for small fires.
Which indicator is used in estimation of bicarbonate?
Methyl Orange is a common indicator used in bicarbonate estimation as it changes color indicating the endpoint of the titration.
What is the formula for bicarbonate formation?
Bicarbonate formation formula is CO2 + H2O → H2CO3 → H+ + HCO3-, showing how carbon dioxide reacts with water forming bicarbonate ions and hydrogen ions.
Is bicarbonate an acid or base?
Bicarbonate (HCO3-) acts as a base when reacting with acids, as it accepts hydrogen ions making the solution less acidic.
What is a bicarbonate example?
A bicarbonate example is Sodium Bicarbonate (NaHCO3), often used as baking soda, showcasing bicarbonate's utility in everyday life.
What is potassium bicarbonate formula?
Potassium Bicarbonate formula is KHCO3, a compound similar to sodium bicarbonate, but with potassium replacing sodium, used in agriculture and medicine.









