Table of Contents
Rules For Filling Electrons in Orbitals – Aufbau Principle: As per Aufbau principle, are there rules for filling electrons in orbitals? If yes, why is this significant? What are the salient features of Aufbau principle? Well, this article answers all your questions. Keep reading below for the same.
The electronic configuration of a molecule refers to the dispersion of electrons in separate molecular orbitals. It is critical to comprehend the molecule. The electronic configuration of a molecule or molecular ion can be used to calculate the number of electrons in its bonding and antibonding molecular orbitals. The stability of an atom can be predicted using electron configuration. Each shell has a fixed number of electrons. It is determined by a simple formula, where the maximum number of electrons for the nth shell is 2n2.
Once an atom occupies all of its orbitals, it becomes the most stable and consequently unreactive. Furthermore, the most stable electron configuration possesses a complete energy state. Noble gasses do not easily combine with other molecules since these orbital configurations are significant features. Unlike many other chemistry concepts, Aufbau is a German word that means “building up.” It is not the name of a scientist. This principle is primarily concerned with the filling of electrons in an orbital during the composition of an electronic configuration.
Overview of Aufbau Principle
Electrons are much smaller than protons and neutrons, weighing over 1,800 times less than either. The electron configuration of an atom is the orbital description of the electron locations in a typical atom. Chemists can anticipate an atom’s attributes, such as stability, boiling temperature, and conductivity, using the electron configuration and physical principles. It is the method or distribution of electrons in an atom’s orbitals. An atom is made up of subatomic particles such as electrons, protons, and neutrons, with only the number of electrons being taken into account for electronic arrangement. Electrons are supplied in such a way that a high constant configuration is achieved.
The quantum property of electrons is electron spin. It is an example of angular momentum. This angular momentum’s magnitude value is fixed. Spin, like charge and rest mass, is a fundamental, unchanging feature of the electron. The spin angular momentum associated with electron spin is distinct from the orbital angular momentum associated with electrons traveling around the nucleus.
Electron Filling Rules
The Hund’s Rule, and the Pauli-Exclusion Principle are a collection of generic rules used to determine the electron configuration of an atomic species. Before proceeding, it is critical to understand that each orbital might be occupied by two electrons with opposing spins (which will be further discussed later).
Rules For Filling Electrons In Orbitals: Aufbau Principle
In general, the Aufbau principle governs how electrons are filled in an atom’s atomic orbitals in its ground state. It states that electrons are filled into atomic orbitals in the sequence of increasing orbital energy levels. We can state the Aufbau principle as the available atomic orbitals with the lowest energy levels occupied first, followed by those with higher energy levels.
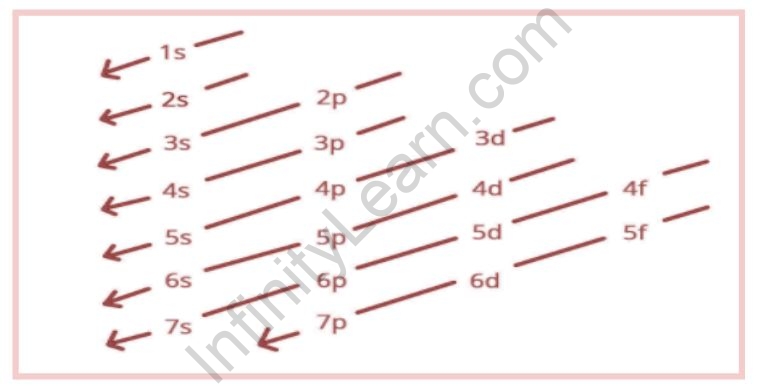
The term “Aufbau” has German roots and can be roughly translated as “construct” or “build-up.” The graphic below depicts the order in which atomic orbitals are filled. The primary quantum number is denoted by ‘n,’ and the azimuthal quantum number is denoted by ‘l.’
The Aufbau principle could be used to explain the placement of electrons in an atom and the energy levels associated with them. Carbon, for example, has 6 electrons and its electrical configuration is 1s22s22p2.
There are numerous outliers, such as chromium’s electron configuration. The half-filled subshell increases orbital stability by exhibiting electron repulsion. Similarly, the full subshell boosts the atom’s stability. This is due to the fact that many atomic electronic structures, such as copper, do not adhere to the Aufbau principle.
Aufbau Principle Order
This order of occupation generally represents the orbitals’ rising energy level. As a result, electrons occupy orbitals in such a way that energy is kept to a minimum. That is, unless the lower energy orbitals, 1s to 6p, are already fully occupied, the 7s, 5f, 6d, and 7p subshells will not be filled with electrons. It is also worth noting that, despite the fact that the energy of the 3d orbital is lower than that of the 4s orbital, electrons occupy the 4s orbital first, followed by the 3d orbital. This discovery can be attributed to the fact that 3d electrons are more likely to be found near the nucleus and so repel each other more strongly.
Salient Features of the Aufbau Principle
- As per the Aufbau’s principle, electrons occupy the orbitals with the lowest energy first. This means that electrons enter higher-energy orbitals only after lower-energy orbitals have been entirely filled.
- The (n+l) rule can be used to identify the sequence in which the energy of orbitals grows, where the sum of the primary and azimuthal quantum numbers determines the energy level of the orbital.
- Lower (n+l) values indicate lower orbital energy. If two orbitals have equivalent (n+l) values, the orbital with the lower n value is said to have lower energy.
- Each and every orbital is filled with electrons in the order: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, and so on.
Aufbau principle – General Concept of Orbitals and their Quantum Numbers
The Aufbau principle establishes some fundamental criteria for filling orbitals in an atom. An atom in its ground state has the least amount of energy and is the most stable. The Aufbau principle governs the filling of orbitals in the ground state of the atom. This principle is also based on the Pauli exclusion principle, Hund’s rule of maximum multiplicity, and the orbital relative energies. According to the principle, electrons are introduced to the various orbitals in increasing energy order. Electrons first enter the lowest energy orbital available to them, then go on to higher energy orbitals only once the lower energy orbitals have been filled.
The quantum mechanical model identifies specific locations in space around the nucleus where the probability of detecting the nucleus is greatest. Such regions are described mathematically and are known as orbital wave functions or simply orbitals. An atom has a massive number of orbitals. The shape, size, and direction of these orbitals quantitatively separate them from one another. A smaller orbital suggests that the electron is more likely to be found near the nucleus. As a result, the Aufbau principle establishes some important guidelines for filling orbitals in an atom.
The information about the Aufbau principle from various chemistry-related articles are available here. The Aufbau principle is an important topic in chemistry. Students who want to flourish in chemistry need to be well known about this to get deep knowledge about it to do well on their exams. The concepts and brief explanations are provided here to assist students in effectively understanding the respective topic.
Get the most Important Questions in Physics, Chemistry, Math, and Biology.
Crack NEET with Result-Oriented Learning Program from Infinity Learn.
Frequently Asked Questions
Is the Aufbau principle true?
The Aufbau principle is used to anticipate the electronic configurations of atoms and, as a result, to explain the periodic table's architecture and how electrons are ordered from low to high energy levels. This is an important topic to remember when teaching physical chemistry. However, the version of this method that has been taught to students is profoundly flawed and unsatisfactory because it also relies on other ideas.
Which elements are exceptional of the Aufbau principle?
Not every element is covered by the Aufbau principle. Ruthenium, rhodium, silver, and platinum, for example, are all exceptions to the Aufbau principle due to filled or half-filled subshells.
What does the l denote in the n+l rule?
Both quantum numbers n and l in the (n + l) rule are used to specify the state of an atom's electron orbital. Here, n signifies the primary quantum number, which is related to the size of the orbital, and l means the angular momentum quantum number, which is related to the form of the orbital.
Infinity Learn App
Now you can find answers to all your subject queries & prepare for your Exams on our Online Education App – Infinity Learn.









