Table of Contents
Introduction
Calcium acetate, also known as calcium ethanoate, is a chemical compound with the formula Ca(C2H3O2)2. Here is some information about calcium acetate. Introduction
Calcium acetate is a chemical compound with the formula Ca(C2H3O2)2. It is derived from the combination of calcium ions (Ca2+) and acetate ions (C2H3O2-). Calcium acetate is commonly used as a food additive, medication, and laboratory reagent
Calcium acetate is a white crystalline powder that is soluble in water. It is produced by reacting calcium carbonate or calcium hydroxide with acetic acid or vinegar. The compound has various applications due to its ability to supply both calcium and acetate ions
In the food industry, calcium acetate is used as a food preservative, acidity regulator, and stabilizer. It helps prevent spoilage in certain food products and assists in maintaining the desired pH levels. Calcium acetate is also used as a dietary supplement to provide calcium to individuals who have calcium deficiencies
In medicine, calcium acetate is prescribed as a phosphate binder. It is used to reduce the levels of phosphates in the blood of patients with kidney disease. By binding to phosphates, calcium acetate helps prevent their absorption in the gastrointestinal tract
Formula of Calcium acetate
Ca(C2H3O2)2
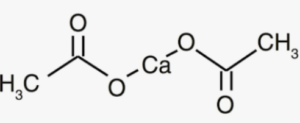
Structure of Calcium acetate
Calcium acetate consists of a calcium cation (Ca2+) and two acetate anions (C2H3O2-). The acetate anion is composed of two carbon atoms, three hydrogen atoms, and two oxygen atoms.
Physical Properties of Calcium acetate
– Appearance: Calcium acetate is a white crystalline powder.
– Melting Point: The melting point of calcium acetate is approximately 160-175 °C.
– Solubility: Calcium acetate is soluble in water.
Chemical Properties of Calcium acetate
– Acidity: Calcium acetate is a weak acid salt and can release acetate ions, which can act as a weak acid in certain reactions.
– Reaction with Water: Calcium acetate readily dissolves in water, forming a clear solution.
– Decomposition: Upon heating, calcium acetate decomposes, releasing acetic acid and leaving behind calcium carbonate.
Uses of Calcium acetate
– Medical Applications: Calcium acetate is commonly used as a medication for the treatment of hyperphosphatemia (high levels of phosphate in the blood) in individuals with kidney disease.
– Food Additive: Calcium acetate is used as a food additive, functioning as a preservative, pH regulator, and firming agent in various food products.
– Laboratory Reagent: Calcium acetate is used as a reagent in laboratory settings for various chemical reactions and experiments.
Conclusion
In conclusion, calcium acetate, with the chemical formula Ca(C2H3O2)2, is a versatile compound used in various industries. It is produced by combining calcium ions and acetate ions. Calcium acetate serves as a food additive, medication, and laboratory reagent.
As a food additive, calcium acetate functions as a preservative, acidity regulator, and stabilizer. It helps extend the shelf life of certain food products and maintains the desired pH levels. In medicine, calcium acetate is prescribed as a phosphate binder to reduce phosphate levels in the blood of individuals with kidney disease. Additionally, calcium acetate is used as a dietary supplement to provide calcium to individuals with calcium deficiencies.
Overall, calcium acetate plays important roles in food production, healthcare, and scientific research, making it a valuable compound with diverse applications.
Solved Example of Calcium acetate
Example 1: Calculate the molar mass of calcium acetate (Ca(C2H3O2)2).
Solution:
To calculate the molar mass, we need to sum up the atomic masses of all the atoms in the formula.
Ca: Atomic mass of calcium = 40.08 g/mol
C2H3O2: Atomic mass of carbon = 12.01 g/mol
Atomic mass of hydrogen = 1.01 g/mol
Atomic mass of oxygen = 16.00 g/mol
Molar mass of calcium acetate (Ca(C2H3O2)2) = 40.08 g/mol + 2 * (12.01 g/mol + 3 * 1.01 g/mol + 2 * 16.00 g/mol)
= 40.08 g/mol + 2 * (12.01 g/mol + 3.03 g/mol + 32.00 g/mol)
= 40.08 g/mol + 2 * (47.04 g/mol)
= 40.08 g/mol + 94.08 g/mol
= 134.16 g/mol
Therefore, the molar mass of calcium acetate is 134.16 g/mol.
Example 2: What is the percent composition of calcium in calcium acetate (Ca(C2H3O2)2)?
Solution:
To find the percent composition of calcium, we need to determine the molar mass of calcium and the molar mass of calcium acetate.
Molar mass of calcium = 40.08 g/mol
Molar mass of calcium acetate = 134.16 g/mol (calculated in Example 1)
Percent composition of calcium = (Molar mass of calcium / Molar mass of calcium acetate) * 100
= (40.08 g/mol / 134.16 g/mol) * 100
= 29.92%
Therefore, the percent composition of calcium in calcium acetate is approximately 29.92%.
Frequently Asked Questions of Calcium Acetate Formula
Is calcium acetate safe for consumption?
Yes, calcium acetate is considered safe for consumption when used as directed. It is commonly used as a food additive and as a medication to treat high blood phosphate levels in certain medical conditions.
What is the solubility of calcium acetate?
Calcium acetate is moderately soluble in water. It dissolves readily in water, forming a clear, colorless solution.
How is calcium acetate used in food?
Calcium acetate is used as a food additive, particularly in certain food products that require a calcium supplement. It can be added to baked goods, dairy products, beverages, and other food items.
What are the benefits of taking calcium acetate as a medication?
Calcium acetate is prescribed as a medication to lower high blood phosphate levels in patients with kidney disease. By binding to phosphate in the body, calcium acetate helps reduce phosphate absorption and maintain phosphate balance in the bloodstream.
Can calcium acetate be used for other purposes besides medication and food?
Yes, calcium acetate has applications beyond medication and food. It is also used in various industrial processes, such as wastewater treatment, as a buffering agent, and as a catalyst in certain chemical reactions.
Is calcium acetate a vitamin?
No, calcium acetate is not a vitamin. It is a compound composed of calcium ions and acetate ions. While calcium is an essential mineral for the body, calcium acetate is not classified as a vitamin.
Is calcium acetate a formula?
Yes, calcium acetate is a chemical formula. The formula Ca(C2H3O2)2 represents the composition of calcium acetate, indicating the presence of calcium ions (Ca2+) and acetate ions (C2H3O2-) in the compound.
Is calcium acetate a fertilizer?
Calcium acetate is not typically used as a fertilizer. It is primarily utilized in the food industry as a food additive, as a medication in the medical field, and as a laboratory reagent in scientific research. In agriculture, other forms of calcium compounds, such as calcium carbonate or calcium nitrate, are commonly used as fertilizers to supply plants with calcium nutrients.




