Table of Contents
Table of contents
- Covalent Bonds formed by Carbon
- Catenation
- Summary
- What’s Next?
In the previous segment, we learnt about Covalent bonds. In this segment, we will learn about the bonds formed by carbon.
What are the Different covalent bonds formed by carbon?
A carbon atom has four valence electrons. So, the sharing of all four electrons is possible. Thus, the valency of carbon is the most important factor for its capacity to form bonds.
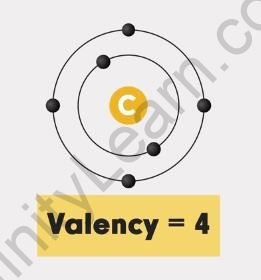
Carbon atom
Carbon atoms can form single, double, or triple covalent bonds.
- Single covalent bonds
For example,
in a methane molecule (\[ CH_{4}\]) a single carbon atom shares its four valence electrons with four different hydrogen atoms. Hydrogen is a monovalent element (only one electron in its outermost shell).

