Table of Contents
What are Covalent bonds?
The bond formed between atoms by the sharing of electrons is known as a Covalent bond.
| S.NO | CONTENT |
| 1 | Covalent Bonds |
| 2 | Types of covalent bonds |
| 3 | covalent bonds formed by carbon |
| 4 | Summary |
| 5 | Did you know |
How are Covalent bonds formed?
- The electronic configuration of carbon is 2, 4. So, it can either donate or accept four electrons to achieve a stable state.
- To achieve stability, carbon opts to share electrons resulting in the formation of covalent bonds.
- When two atoms form a covalent bond, both need to contribute to the bond formation. This means each atom has to contribute at least one electron to form the bond.
- So a covalent bond has at least one pair of shared electrons.
What are the Different types of covalent bonds?
Based on how many pairs of electrons are shared between the atoms, there are three types of covalent bonds: single covalent bond, double covalent bond, and triple covalent bond.
What is single covalent bond?
A covalent bond in which One pair of electrons is shared between the atoms is called a single covalent bond. It is denoted by drawing a single line between the two atoms.
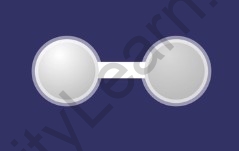
Single covalent bond
For example,
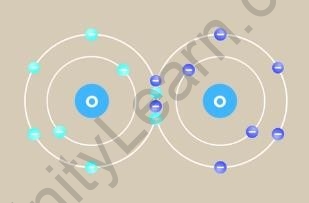
a chlorine atom has seven electrons in its outermost shell. That means only one electron is needed by it to complete the octet. So it shares one electron with another chlorine atom to form a chlorine molecule.
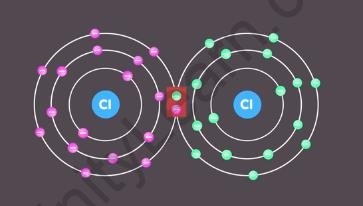
Chlorine molecule formed by sharing of 2 electrons
What is double covalent bond?
A covalent bond in which Two pairs of electrons is shared between the atoms is called a double covalent bond. It is denoted by drawing two lines between the two atoms.
For more visit Polar covalent bond



