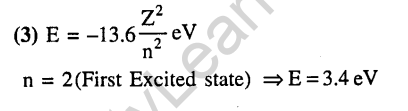JEE Main Previous Year Papers Questions With Solutions Chemistry Atomic Structure and Electronic Configuration
1.In a hydrogen atom, if energy of an electron in the ground state is 13.6 eV, then that in the 2nd excited state is (2002)
1) 1.51 eV 2) 3.4 eV 3) 6.04 eV 4)13.6 eV
Ans.
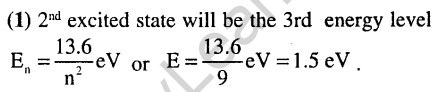
2.
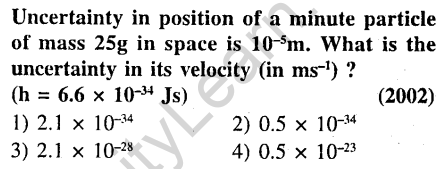
Ans.
Join Our Courses: JEE Class 11 Students | JEE Class 12 Students | JEE Dropper
3.Which of the following ions has the maximum magnetic moment. (2002)
1) Mn+2 2) Fe+2 3) Ti+2 4) Cr+2
Ans.(1) Mn2+ has the maximum number of unpaired electrons (5) and therefore has maximum moment
4.The number of d-electrons retained in Fe2+ (At.no. of Fe = 26) ion is (2003)
1) 4 2) 5 3) 6 4) 3
Ans.(3) Configuration of Fe+2 = 3d6.4s°
5.
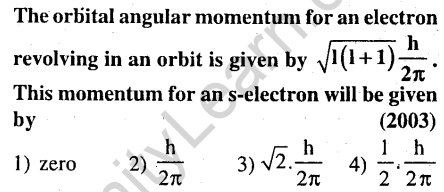
Ans.(1) For s-electron, l = 0; Angular momentum=zero
6.In Bohr series of lines of hydrogen spectrum, the third line from the red end corresponds to which one of the following inter-orbit jumps of the electron for Bohr orbits in an atom of hydrogen (2003)
1) 5 —» 2 2)4 —>1 3) 2—>5 4) 3->2
Ans.

7.
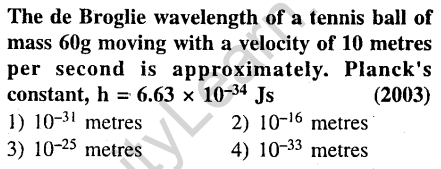
Ans.
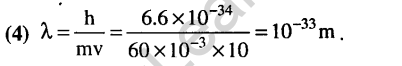
8.
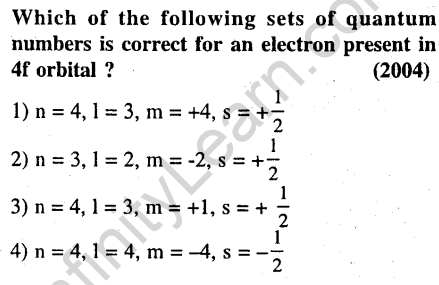
Ans.(3) For 4f orbital, n= 4 and 1 = 3. Values of m = -3, -2, -1,0, +1, +2, +3. Combination of 1 = 3 m = +1 is the only acceptable option.
9.Consider the ground state of Cr atom (Z=24).The number of ■electrons with the azimuthal quantum numbers 1 =1 and 2 are respectively
1)12 and 4 2) 16 and 5 (2004)
3) 16 and 4 4) 12 and 5
Ans.(4) Configuration of Cr (Z = 24) is ls22s22p63s23p63d54s*
1=1, denotes p-electrons (2p6, 3p6)
1 = 2,’denotes d-electrons (3d5)
10.
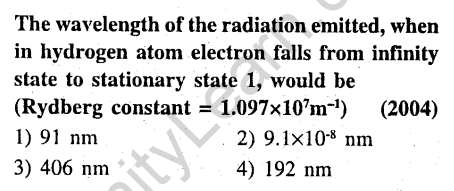
Ans.
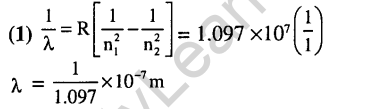
11.

Ans.(2) Like +7 state for Mn
12.In a multi-electron atom, which of the following orbitals described by the three quantum numbers will have the same energy in the absence of magnetic and electric fields?(2005)
(a) n = 1,1 = 0, m = 0 (b) n = 2,1 = 0, m = 0
(c) n = 2,1 = 1, m = 1 (d) n = 3,1 = 2, m = 1 (e) n = 3,1 = 2, m = 0
1) (a) and (b) 2) (b) and (c)
3) (c) and (d) 4) (d) and (e)
Ans.(4) n is same and orbital is 3d.
13.Which of the following statements in relation to the hydrogen atom is correct ? (2005)
(1)3s orbital is lower in energy than 3p orbital
(2)3p orbital is lower in energy than 3d orbital
(3)3s and 3p orbitals are of lower energy than 3d orbital
(4)3s, 3p and 3d orbitals all have the same energy
Ans.4) Auf-bau principle is not applicable for H atom

Ans.
![]()
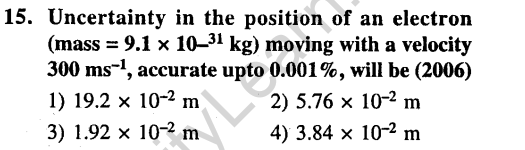
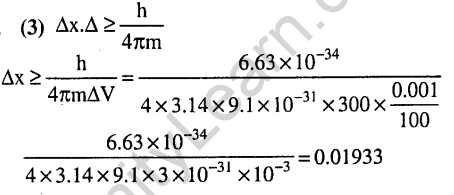
Ans.
16.The “spin-only” magnetic moment [in units of Bohr magneton] of Ni2+ in aqueous solution would be (Atomic number of Ni = 28) (2006)
1) 2.84 2) 4.9 3) 0 4) 1.73
Ans.

17.Which of the following sets of quantum numbers represents the highest energy of an atom ? (2007)
(1)n=3, 1=2, m=l, s=+l/2
(2)n=3, 1=2, m=l, s=+l/2
(3)n=4, 1=0, m=0, s=+l/2
(4)n=3, 1=0, m=0, s= +1/2
Ans.(2) Maximum value of (n +1) denotes highest energy of the orbital.
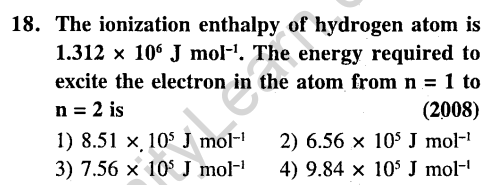
Ans.
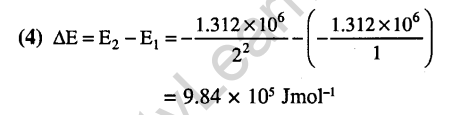
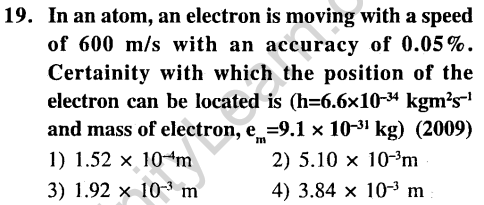
Ans.
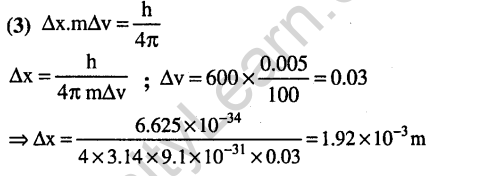
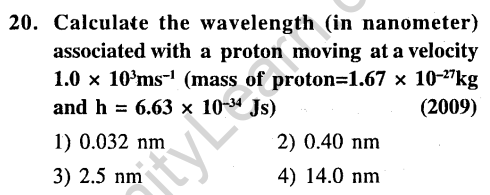
Ans.
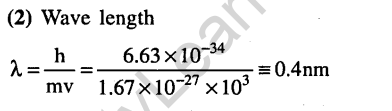
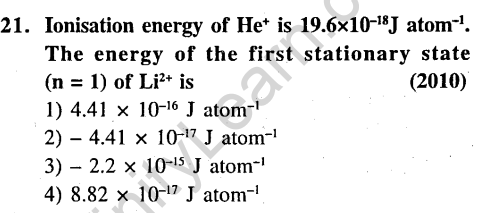
Ans.
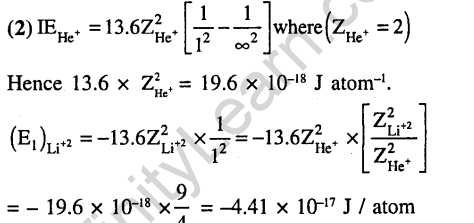

Ans.

23.A gas absorbs a photon of 355 nm and emits
at two wavelengths. It one of the emissions is at 680 nm, the other is at (2011)
1) 743 2) 518 3) 1035 4) 325 nm
Ans.
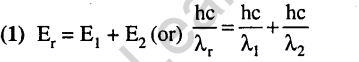
24.The electrons identified by quantum numbers n and l (2012)
a) n = 4, / = 1 b) n = 4, l = 0
c) n = 3, l = 2 d) n = 3, / = 1
can be placed in order of increasing energy as
l)c<d<b<a 2)d<b<c<a
3)b<d<a<a 4)a<c<b<d
Ans.(2) Greater the (n + /) value, greater is the energy. If (n + l) values are equal, then greater the ‘n’ value greater is the energy.
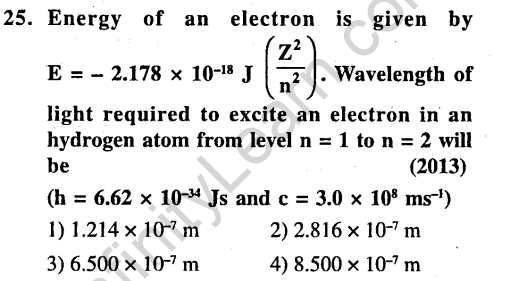
Ans.

Ans.(2) 5s1 configuration of valence electron.
27.Which of the following is the energy of a possible excited state of a possible excited state of hydrogen? (2015)
1) +13.6 eV 2) -6.89 eV
3) -3.4 eV 4) +6.8 eV
Ans.