JEE Main Previous Year Papers Questions With Solutions Chemistry Chemical and Iconic Equilibrium
1.M NaCl and 1 M HC1 are present in an aqueous solution. The solution is (2002)
1)not a buffer solution with pH < 7
2)not a buffer solution with pH > 7
3)a buffer solution with pH < 7
4)a buffer solution with pH > 7
Ans.(1) A buffer is a solution of weak acid and its salt with strong base and vice versa. HCl is strong acid and NaCl is its salt with strong base. pH is less than 7 due to HCl.
2.Species acting as both Bronsted acid and base is (2002)
1)HS04-1 2) Na2C03 3) NH3 4) OH-1
Ans.
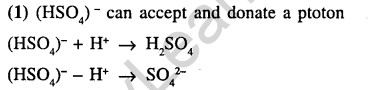
3.Let the solubility of an aqueous solution of Mg(OH)2 be x, then its ksp is (2002)
1)4x3 2) 108x5 3) 27x4 4) 9x
Ans.
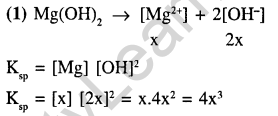
4.The solubility of Mg(OH)2 is S moles / litre. The solubility product under the same condition is (2002)
1)4S3 2) 3S4 3) 4S2 4) S3
Ans.
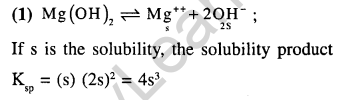
5.Change in volume of the system does not alter the number of moles in which of the following equilibria ? (2002)
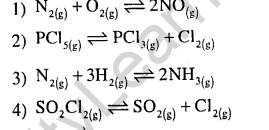
Ans.(1) In this reaction the ratio of number of moles of reactants to products is same, hence change l in volume will not alter the number of moles
6.In which of the following reactions, increase in the volume at constant temperature does not affect the number of moles at equilibrium(2002)
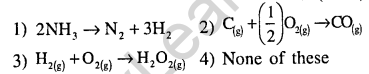
Ans.
Ans.
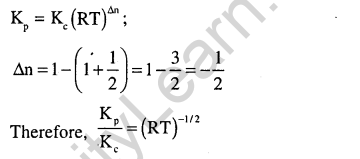
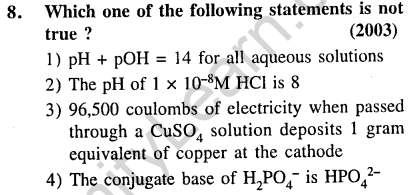
Ans.(2) An acidic solution cannot have a pH > 7.
9.The solubility in water of a sparingly soluble salt AB2 is 1.0 x 10-5 molL”1. Its solubility product number will be (2003)
1)4 x 10-10 2) 1 x 10-15
3) 1 x 10-10 4) 4 x 10-15
Ans.
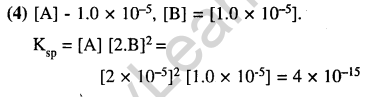
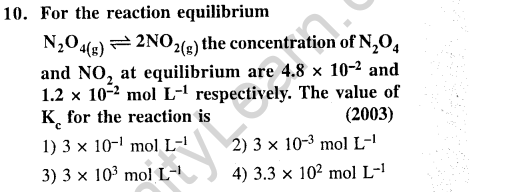
Ans.
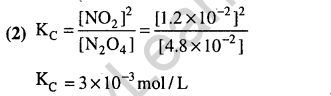
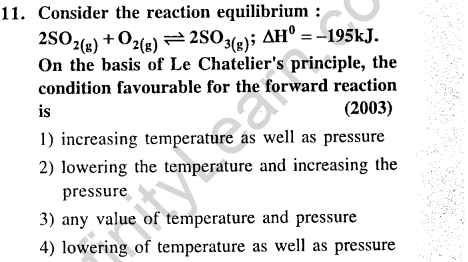
Ans.(2) Due to exothermicity of reaction low or optimum temperature will be required. Since 3 v moles are changing to 2 moles. High pressure will be required.
12.Which one of the following substances has the highest proton affinity ? (2003)
1)H2S 2) NH3 3) PH3 4) H20
Ans.(2) H2S is more acidic.
13.In a 0.2 molal aqueous solution of a weak acid HX the degree of ionization is 0.3. Taking kf for water as 1.85, the freezing point of the solution will be nearest to (2003)
1) – 0.360°C 2) – 0.260°C
3) + 0.480°C 4) – 0.480°C
Ans.(4) Decrease by 1.85 x 0.3 x 1.3 = 0.48°C
14.The conjugate base of H2P04– is (2004)
1) P043– 2) HP042- 3)H3P04 4)P205
Ans.
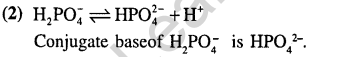
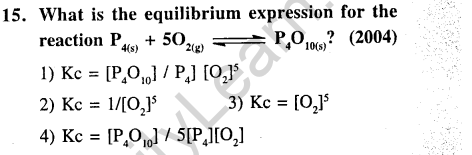
Ans.(2) Applying law of mass action, the expression for K is obtained. Only 02 is a gas and hence its active mass only appear in K.
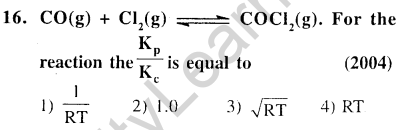
Ans.(1) Kp = Kc (RT)in .Here, An is 1 – (1 + 1) = – 1.
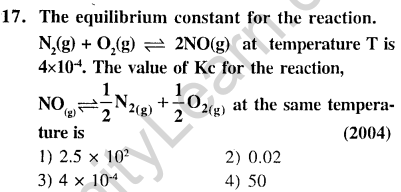
Ans.(4) As per property of equilibria, reverse the equation and divide it by 2.

Ans.(2) If ‘s’ is the solubility Ksp = 108s5 1 x 44 x s1+4 = 256 s5 = Ksp
19.The solubility product of a salt having general formula MX2, in water is : 4xl0-12. The concentration of M2+ ions in the aqueous solution of the salt is (2005)
1) 2.0 x 10.6 M 2) 1.0 x 10.4 M
3) 1.6 x 10.4 M 4) 4.0 x 10.10 M
Ans.
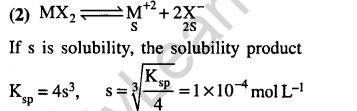
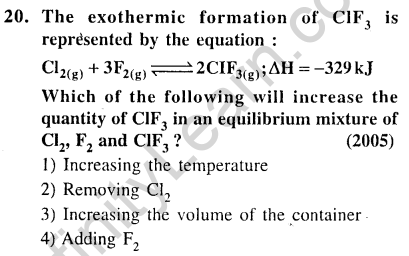
Ans.(4) An increase in the concentration of a reactant at equilibrium, the forward reaction is favoured. Hence, adding F2
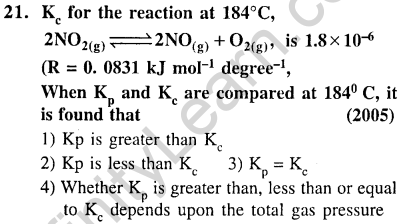
Ans.

22.Hydrogen ion concentration in mol L-1 in a solution of pH = 5.4 will be (2005)
1) 3.98 x 108 2) 3.88 x 106
3) 3.68 x 10-6 4) 3.98 x 10-6
Ans.
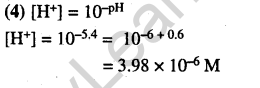
23.What is the conjugate base of OH– ? (2005)
1) 02 2) H20 3) 0– 4) O-2
Ans.
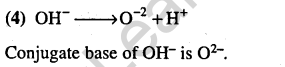
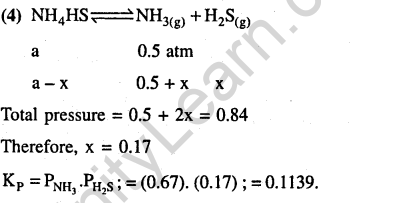
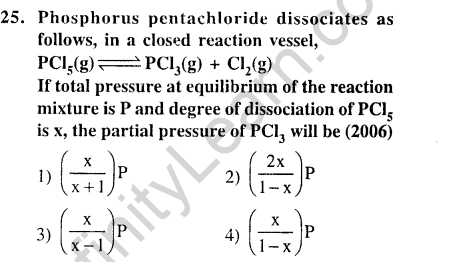
24.An amount of solid NH4HS is placed in a flask already containing ammonia gas at a certain temperature and 0.5 atm. Ammonium hydrogen sulphide decomposes to yield NH3 and 1I2S gases in the flask. When the decomposition reaction reaches equilibrium, the total pressure in the flask rises to 0.84 atm. The equilibrium constant for NII4HS decomposition at this temperature is (2005)
1) 0.30 2) 0.18 3) 0.17 4) 0.11
Ans.
Ans.
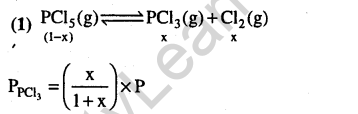
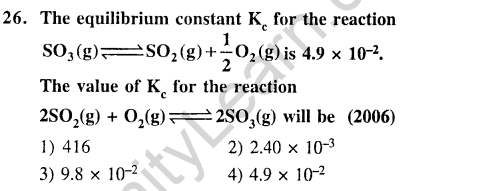
Ans.
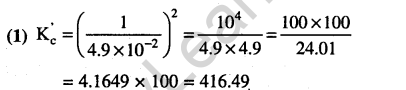
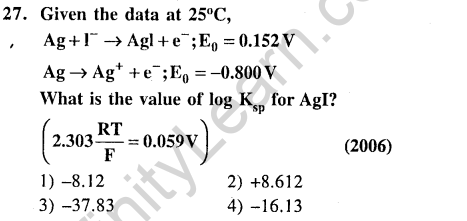
Ans.
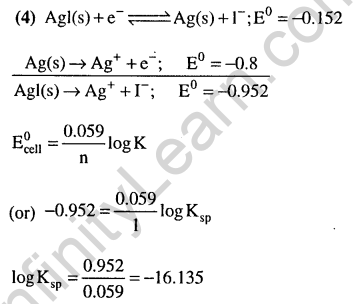

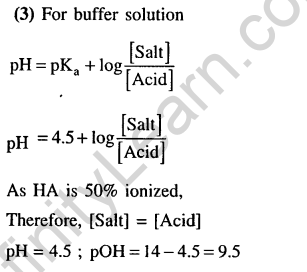
28.The pKa of a weak acid (HA) is 4.5. The pOH of an aqueous buffered solution of HA in which 50% of the acid is ionized is (2007)
1) 4.5 2) 2.5 3) 9.5 4) 7.0
Ans.
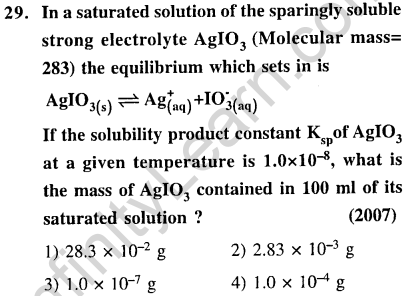
Ans.
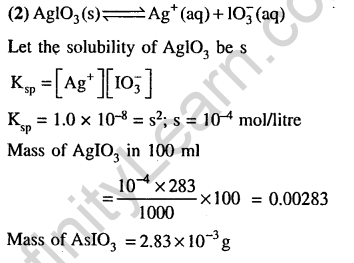
30.The first and second dissociation constants of an acid H2A are 1.0 x 10-5 and 5.0 x 10“10 respectively. The overall dissociation constant of the acid will be (2007)
1) 5.0 x 10-5 2) 5.0 x 1015
3) 5.0 x 10-15 4) 0.0 x 105
Ans.
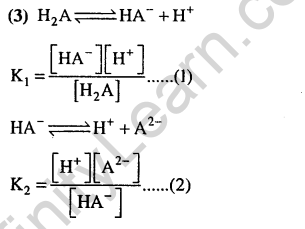
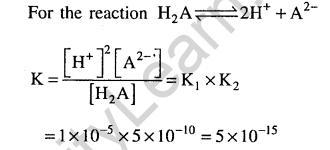

Ans.
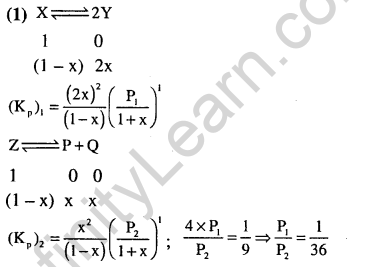
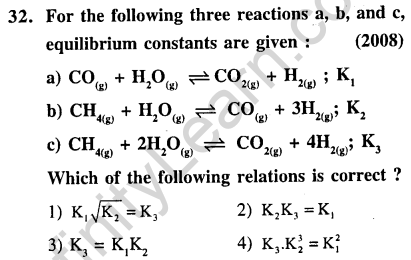
Ans.(3) Equation (c) = equation (a) + equation (b). Thus K, = K,.K2
33.Four species are listed below
i) HC0–3; ii) H30+ iii) HSO–4 iv) HS03F
Which one of the following is the correct sequence of thfflr acid strength ? (2008)
1) iv < ii < iii <i 2) ii < iii < i < iv
3) i < iii < ii < iv 4) iii < i < iv < ii
Ans.
![]()
34.The pKa of a weak acid, HA, is 4.80. The pKb of a weak base, BOH, is 4.78. The pH of an aqueous solution of the corresponding salt, BA, will be (2008)
1) 9.58 2) 4.79 3) 7.01 4) 9.22
Ans.
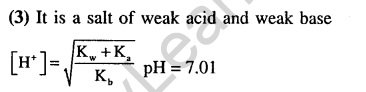
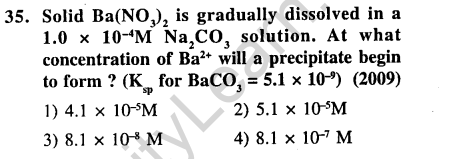
Ans.
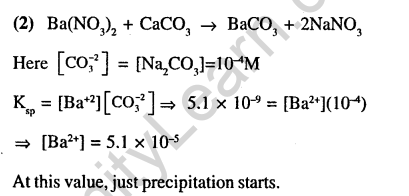
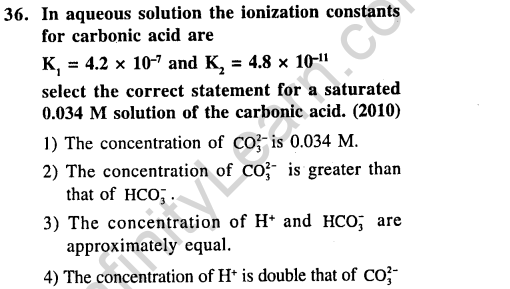
Ans.
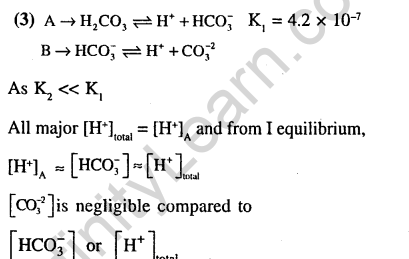
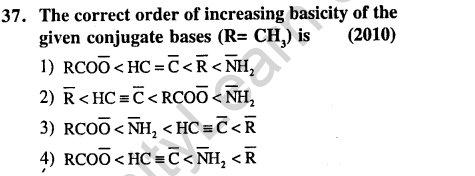
Ans.
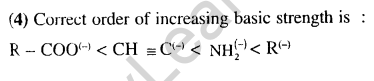
38.Solubility product of silver bromide is 5.0x 10-13. The quantity of potassium bromide (molar mass is 120g of mol-1) to be added to 1 litre of 0.05 M solution of silver nitrate to start the precipitation of AgBr is (2010)
1) 1.2 x 10-10g 2) 1.2 x 10-9g
3) 6.2 x 10-5g 4) 5.0 x 10-8g
Ans.
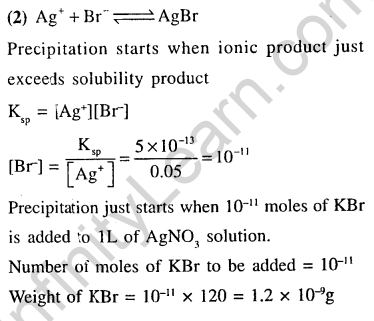
39.At 25°C, the solubility product of Mg(OH)2 is 1.0 10_n. At which pH, will Mg2+ ions start precipitating in the form of Mg(OH)2 from a solution of 0.001 M Mg2+ ions ? (2010)
1)9 2) 10 3)11 4)8
Ans.
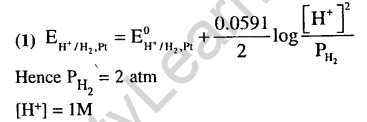
40.The reduction potential of hydrogen half-cell will be negative if : (2011)
1)P(H2) = 2 atm and [H+] = 1.0 M
2)P(H2) = 2 atm and [H+] = 2.0 M l
3)P(H2) = 1 atm and [H+] = 2.0 M
4)P(H2) = 1 atm and [H+] = 1.0 M
Ans.
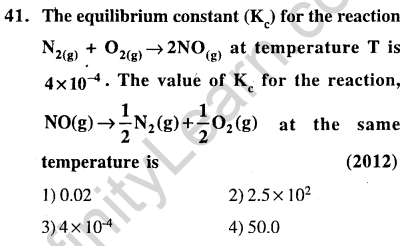
Ans.
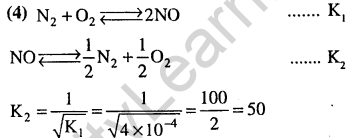
42.The pH of a 0.1 molar solution of the acid HQ is 3. The value of the ionization constant Ka of this acid is (2012)
1) 3×10_1 2) 1×10-3
3) 1×10-5 4) 1×10-7
Ans.

Ans.(4) If SRP value is high-powerful oxidising agent.

Ans.
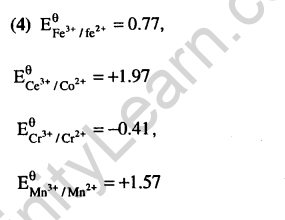
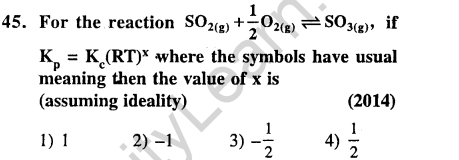
Ans.
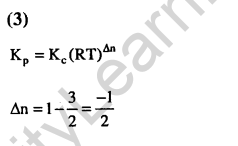
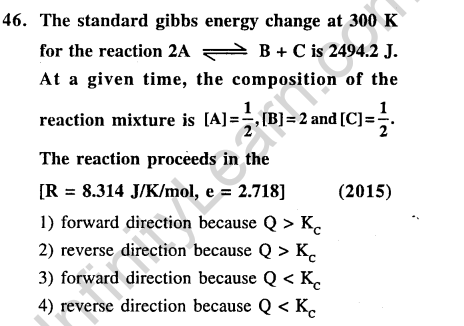
Ans.