To Prepare a Pure Sample of Potash Alum (Fitkari), [K2SO4.Al2 (SO4)3. 24H20]
Theory
Potash alum is prepared by dissolving an equimolar mixture of hydrated aluminium sulphate and potassium sulphate in minimum amount of water containing a little of sulphuric acid and then subjecting the resulting solution to crystallisation, when octahedral crystals of potash alum separate out.
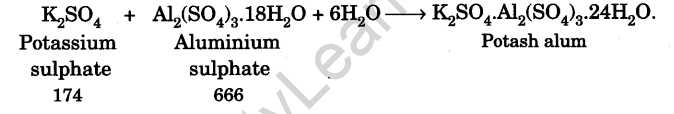
Requirements
Two beakers (250 ml), china-dish, funnel, funnel-stand, glass-rod, wash-bottle, tripod stand and wire-gauze. Potassium sulphate, aluminium sulphate and dil. sulphuric acid.
Procedure
- Take a 250 ml beaker. Wash it with water and then transfer 2.5 g potassium sulphate crystals to it. Add about 20 ml of water. Stir to dissolve the crystals. Warm if required.
- Take the other 250 ml beaker, wash it with water and then transfer 10 g aluminium sulphate crystals to it. Add about 20 ml of water and 1 ml of dilute sulphuric add to prevent hydrolysis of aluminium sulphate. Heat for about 5 minutes. If milkiness still persists, filter the solution.
- Mix the two solutions in a china-dish and place the china-dish on a wire-gauze placed over a burner. Stir the solution with a glass-rod. Concentrate the solution till the crystallisation point is reached. Place the dish over a beaker containing cold water.
- Soon the crystals of potash alum separate out. Decant off the mother liquor and wash the crystals with a small quantity of ice-cold water.
- Dry the crystals by placing them between filter paper pads or by spreading them over porous plate.
Observations
Weight of crystals obtained = …………g
Expected yield = …………g
Colour of the crystals =…..
Shape of the crystals = ………
Note: The crystals of potash alum are octahedral in shape.
Precautions
- Cool the solution slowly to get good crystals.
- Do not disturb the solution while it is being cooled.




