Viva Questions with Answers
Question.1.What is qualitative analysis?
Answer. The type of analysis that deals with the methods which are used to determine the constituents of a compound.
Question.2.What is a radical?
Answer. A radical may be defined as an atom or group of atoms which carries charge and behaves as a single unit in chemical reactions.
Question.3. What are acidic and basic radicals?
Answer. Radicals carrying positive charge are called basic radicals and those carrying negative charge are called acidic radicals.
Question.4. What type of bond is present in an inorganic salt?
Answer. Electrovalent bond.
Question.5. Why do inorganic salt ionizes when dissolved in water?
Answer.Due to the high dielectric constant of water, the force of attraction holding the two ions in a salt decreases. Thus, the two ions separate. The ions are ‘further stabilized by solvation.
Question.6. Name the coloured basic radicals.
Answer. Cu2+, Fe2+, Fe3+, Cr3+, Ni2+, Co2+ and Mn2+.
Question.7. What is the colour of iron salts?
Answer. Ferrous salts are usually light green while ferric salts are generally brown.
Question.8. Name any iron salt which is light green.
Answer. Ferrous sulphate.
Question.9. What is the colour of nickel salts?
Answer. Bluish green or green.
Question.10. What is the colour of manganese salts?
Answer. Light pink or flesh colour.
Question.11. Name the basic radicals which are absent, if the given salt is white.
Answer. Cu2+, Fe2+, Fe3+, Cr3+, Ni2+, Co2+ and Mn2+.
Question.12. Why a salt containing lead turn black in colour, when placed for a long time in laboratory ?
Answer. Due to the formation of black lead-sulphide by the action of H2S in atmosphere.
Question.13. Name the salts which produce crackling sound when heated.
Answer. Lead nitrate, barium nitrate, potassium bromide, sodium chloride.
Question.14. What is sublimation?
Answer. It is the process by which a salt directly changes into gaseous phase without melting, when heated. On cooling vapours condense back to the solid state.
Question.15. Tell the importance of preliminary tests in qualitative analysis.
Answer. Sometimes, preliminary tests give authentic information about an ion in the salt. For example, golden yellow colour in flame test shows the presence of sodium. In a charcoal cavity test, brown residue shows the presence of cadmium in a salt and so on.
Question.16. How is dry heating test performed and what information you get if the residue changes to yellow when hot?
Answer. In dry heating test, the salt is heated in a dry test tube. Yellow residue when hot shows the presence of zinc.
Question.17. What is the expected information when copper sulphate is heated in a dry test tube?
Answer. A white residue is formed and water condenses on the colder walls of the test tube.
Question.18. Name the radical which produces CO2 on heating.
Answer. Carbonate.
Question.19. What is the colour of residue when zinc salt is heated?
Answer. A residue yellow when hot and white when cold is formed.
Question.20. What is the colour of residue when cadmium salt is heated?
Answer. A residue brown when hot, brown when cold.
Question.21. If the residue in dry heating test is white, name’the radicals which are absent.
Answer. Cu2+, Fe2+, Ni2+, Mn2+ Co2+, Cr3+, Cd2+, Zn2+ and Pb2+.
Question.22. How is charcoal cavity test performed? Describe the chemistry for the formation of incrustation as well as metallic bead.
Answer. The salt is mixed with the double the quantity of sodium carbonate and the mixture is heated in the charcoal cavity in luminous flame (reducing flame).
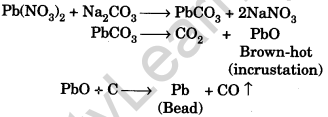
Question.23. Which flame is used in charcoal cavity test? How is it obtained?
Answer. A reducing flame is used in charcoal cavity test. It is obtained by closing the air holes of the Bunsen burner.
Question.24. Why should we avoid excess of cobalt nitrate in cobalt nitrate test?
Answer. Excess of cobalt nitrate is avoided because it forms black cobalt oxide in the oxidising flame. This colour masks the other colours which might be produced during the test.
Question.25. In the flame test, sodium imparts yellow colour to the flame while magnesium does not impart any colour. Why?
Answer. In case of magnesium, the energy of flame is unable to promote the electron to higher energy level, hence, no colour is imparted to the flame.
Question.26. What is the chemistry of flame test.
Answer. In flame test, the valence electron of the atom gets excited and jumps to the higher level. When the electron jumps back to the ground state, the radiation is emitted whose frequency falls in the visible region.
Question.27. What is the function of blue glass in flame test?
Answer. The blue glass can absorb a part or whole of the coloured light in certain cases. Therefore, the flame appears to be of different colour when viewed through blue glass. This helps in identification of some basic radicals.
Question.28. Why do we use cone. HCl in preparing a paste of the salt for flame test?
Answer. In order to convert metal salts into metal chlorides which are more volatile than other salts.
Question.29. Why can’t we use glass rod instead of platinum wire for performing flame test?
Answer. This is because glass contains sodium silicate which imparts its own golden yellow colour to the flame.
Question.30. Why is platinum metal preferred to other metals for flame test?
Answer. Because platinum does not react with acids and does not itself impart any characteristic colour to the flame.
Question.31. Why do barium salts not impart colour to the flame immediately?
Answer.Because barium chloride is less volatile, it imparts colour to the flame after some time.
Question.32. Why should we avoid the use of platinum wire for testing lead salts?
Answer. Because lead combines with platinum and the wire gets corroded.
Question.33. Why should only a particle or two of the given salt should be touched with the bead in borax bead test?
Answer. If salt is used in excess an opaque bead is formed.
Question.34. Why borax bead test is not applicable in case of white salts?
Answer. White salts do not form coloured meta-borates. .
Question.35. What is Nessler’s Reagent?
Answer. It is a solution of mercuric iodide in potassium iodide. Its formula is K2[HgI4].
Question.36. Name the acid radicals detected with dil. H2SO4?
Answer.
CO32-,S2-,SO32-,NO2-
Question.37. Why dil. H2SO4 is preferred while testing acid radicals over dil. HCl ?
Answer. When the salt is treated with HCl, during reaction HCl gas is also given out along with the gas evolved by the salt. So the actual gas cannot be identified whereas with H2SO4, no such problem arises.
Question.38. Name the acid radicals detected by cone. H2SO4.
Answer.
![]()
Question.39. Name the radicals which are tested with the help of water extract.
Answer. NO3–, NO2– and CH3COO–.
Question.40. Name the radicals which are confirmed with the help of sodium carbonate extract.
Answer.S2-, Cl–, Br–,I–, PO43-, SO42-, SO32-, C2O42-.
Question.41. How is sodium carbonate extract prepared?
Answer. The salt is mixed with double the amount of solid Na2CO3 and about 20 ml of distilled water. It is then boiled till it is reduced to one-third, and then filtered. The filtrate is sodium carbonate extract or (S.E.).
Question.42. What is water extract?
Answer. The given salt or mixture is shaken well with distilled water and the solution is filtered. The filtrate is water extract.
Question.43. CO2 and SO2 both turn lime water milky. How will you distinguish between them?
Answer. By passing through acidified K2Cr2O7 solution. SO2 turns green while CO2 has no effect.
Question.44. NO2 and Br2 both are brown in colour. How will you distinguish between them?
Answer. By passing through FeSO4 solution. NO, turns FeSO4 soln. black while Br2 has no effect.
Question.45. How will you test the presence of carbonate?
Answer. Treat a small quantity of the mixture with dil. H2SO4. CO2 gas is evolved. When the gas is passed through lime water, it is turned milky.
![]()
Question.46. What is lime water?
Answer. A solution of Ca(OH)2 in water is called lime water.
Question.47. What will happen if excess of CO2 is passed through lime water?
Answer. The white ppt. of CaCO3 changes into soluble calcium bicarbonate and the milkiness, therefore, disappears.
![]()
Question.48. How do you test for sulphide?
Answer. Warm the salt with dil. H2SO4. H,S gas is evolved. It turns a paper dipped in lead acetate black.
![]()
Question.49. Is there any gas other than CO2, which turns lime water milky?
Answer. Yes, it is SO2 gas.
Question.50. All nitrates on heating with cone. H2SO4 in presence of paper pallet evolve NO2 gas. What is the function of paper pallet?
Answer.
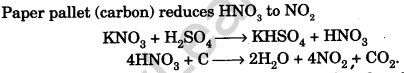
Question.51. How will you test whether the given solution in a bottle is lime water?
Answer. Take 2 ml of the solution in a test tube and blow into it by means of a glass tubing. Milkiness indicates that the solution is lime water.
Question.52. How is ring test performed for nitrates?
Answer. To the salt solution, freshly prepared ferrous sulphate solution is added and then sulphuric acid (cone.) is added along the walls of the tube. A dark brown ring is formed at the junction of the two solutions.
Question.53. Why the hot reaction mixture in case of cone. H2SO4 ( test is not thrown into the sink?
Answer. In order to avoid spurting, due to which H2SO4 may fly and spoil clothes and may result into serious injuries.
Question.54. What is Tollen’s reagent?
Answer. Ammonical AgNO3 solution is called Tollen’s reagent.
Question.55. Give formula of Diphenylamine reagent.
Answer. (C6H5)2 NH.
Question.56. Why a dark brown ring is formed at the junction of two layers in ring test for nitrates?
Answer. H2SO4 being heavier forms the lower layer and reacts only with a small amount of nitrate and FeSO4 at its surface, therefore, a brown ring appears only at the junction of the two layers.
Question.57. Why acetic acid is added before adding lead acetate solution?
Answer. In order to prevent the hydrolysis of lead acetate which would yield white precipitate of lead hydroxide.
Question.58. What is the formula of Sodium nitroprusside?
Answer. Na2[Fe(CN)5 NO].
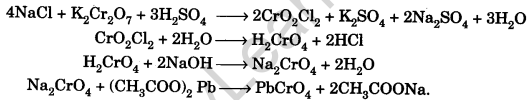
Question.59. What is chromyl chloride test?
Answer. Heat a small amount of the mixture with cone.H2SO4 and solid K2Cr2O7 in a dry test tube. Deep brownish red vapours of chromyl chloride are formed. Pass these vapours in water. A yellow sol. of H2CrO4 is formed. Add to this solution NaOH, acetic acid and lead acetate, a yellow ppt. confirms chloride in the mixture.
Question.60. What is the chemistry of carbon disulphide test for a bromide or iodide?
Answer. To a part of the soda extract add dil. HCl. Now to this add small amount of CS2and excess of chlorine water and shake the solution well. Chlorine displaces bromine or iodine from the bromide or iodide, which dissolves in carbon disulphide to produce orange or violet colouration.
![]()
Question.61. Why do bromides and iodides not respond to chromyl chloride test?
Answer. Because chromyl bromide (CrO2Br2) and chromyl iodide (CrO2 I2) compounds are not formed, instead of these bromine and iodine are evolved.
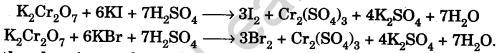
Question.62. Describe the chemistry of match stick test.
Answer.In match stick test, the sulphate is reduced to sulphide by carbon of match stick which then gives violet colour with sodium nitroprusside solution.
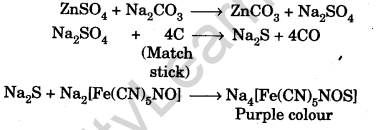
Question.63. Why does iodine give a blue colour with starch solution?
Answer. The blue colour is due to the physical adsorption of iodine upon starch.
Question.64. Why O.S. is not prepared in cone. HNO3?
Answer. HNO3 is an oxidising agent which on decomposition gives oxygen. A yellow ppt. of sulphur is obtained in presence of HNO3 when H2S is passed.
![]()
Question.65. Name group reagents for different groups.
Answer.

Question.66. Why is it essential to add dil. HCl before proceeding to the test for the basic radicals of group II?
Answer. In the precipitation of group II cations as their sulphides. H2S is used in the presence of dil. HCl. H2S is itself a weak acid and dissociates as follows:
![]()
Hydrochloric acid being a strong acid is largely ionised to H+. Thus hydrogen ion concentration is increased and consequently the concentration of sulphide ions produced by the ionisation of H2S is sufficiently decreased due to common ion effect. As a result of which the sulphide ion concentration is sufficient only to exceed the solubility product of the sulphides of group II cations.
Since the solubility products (Ksp) for the sulphides of groups III and IV cations are very high, those cations are not precipitated out under the above conditions.
Question.67. Why is the O.S. boiled with cone. HNO, in III group?
Answer. In the presence of NH4Cl, Fe(OH)2 is not precipitated because of its high solubility product. For this reason Fe++ salts are oxidised to Fe++ salts by boiling with cone. HNO3 before adding NH4Cl and NH4OH; otherwise Fe++ would not be ppted in III group.
![]()
Question.68.Why is NH4Cl added along with NH4OH in III group?
Answer. It is done in order to decrease the concentration of OH– ions by suppressing the ionisation of NH4OH by common ion effect. If NH4OH alone is used in that case, the concentration of OH– is enough to ppt. the hydroxide of IV, V and VI groups.
Question.69.What is blue lake?
Answer. It is blue particles (blue litmus adsorbed on white ppt. of Al(OH)3 floating in colourless solution.
Question.70.H2S gas is passed in presence of NH4OH in group IV. Explain why?
Answer. When H2S gas is passed in alkaline medium or NH4OH, the OH+ ions from the dissociation of H2S gas combine with hydroxyl ions (OH–) from the dissociation of NH4OH to form nearly unionised H2O.
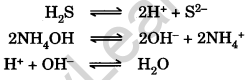
The removal of H+ ions from the solution causes more of H2S to dissociate, thereby increasing the concentration of S2- ions to such an extent that the ionic product of IV group metal sulphides exceeds their solubility product. Hence they are precipitated.
Question.71.Presence of NH4Cl is quite essential before the addition of (NH4)2 CO3 in group V. Explain why?
Answer. Ammonium chloride suppresses the ionisation of NH4OH and (NH4)2CO3 due to common ion effect which results in the decrease in the concentration of OH– and CO32- ions. So the ionic product does not exceed the solubility product of Mg(OH)2 or MgCO, and thereby they are not precipitated in V group.
Question.72.Na,CO, cannot be used in place of (NH4)2 CO, in the group V. Explain why?
Answer. Na2CO3 is highly ionised electrolyte, which produces very high cone, of CO32- ions. As a result ionic product of MgCO3 may increase its Ksp and “it may get precipitated along with the radicals of V group.
Question.73.An aqueous solution of HCl has cone. KP M. What is the approximate value of pH of this solution?
Answer. Slightly less than 7.
Question.74.How will your prepare chlorine water?
Answer. Take cone. HCl in a test tube and add KMnO4 soln. dropwise till the pink colour starts persisting. Now add a few drops of cone. HCl so that pink colour disappears. The colourless solution thus obtained is chlorine water.
Question.75.Can we use ammonium sulphate in place of ammonium chloride in group III precipitation?
Answer. No, ammonium sulphate cannot be used because it would cause precipitation of group V radicals as their sulphates in group III.
Question.76.Name a cation which is not obtained from a metal?
Answer. Ammonium ion (NH4+).


