Theory
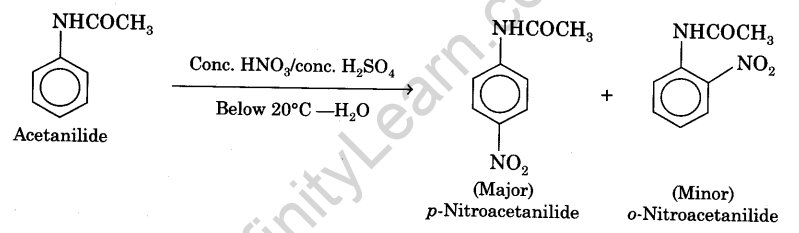
The nitration of aniline is difficult to carry out with nitrating mixture (a mixture of cone. H2SO4 ,and cone. HN03 ) since —NH2 group gets oxidised which is not required. So the amino group is first protected by acylation to form acetanilide which is then nitrated to give p-nitroacetanilide as a major product and o-nitroacetanilide as a minor product. Recrystallisation from ethanol readily removes the more soluble ortho-compound and the pure p-nitroacetanilide is obtained. The chemical equation can be written as :
Apparatus
Conical flask (100 ml), beaker (250 ml), measuring cylinder (100 ml), funnel, glass-rod, test-tube, filter-papers, etc.
Chemicals Required
Acetanilide = 5g
Glacial acetic acid = 5 ml
Cone. H2S04 =10 ml
Fuming HN03 = 2 ml
Methylated spirit = 20 ml.
Procedure
- Take a 100 ml conical flask and add 5 g of powdered acetanilide in it. Add 5 ml of glacial acetic acid and stir the mixture by the use of glass-rod.
- Place 2 ml of fuming nitric acid in a clean test-tube and cool it in a freezing mixture (ice + salt) taken in a beaker. Carefully add drop by drop 2 ml of cone, sulphuric acid with constant shaking and cooling.
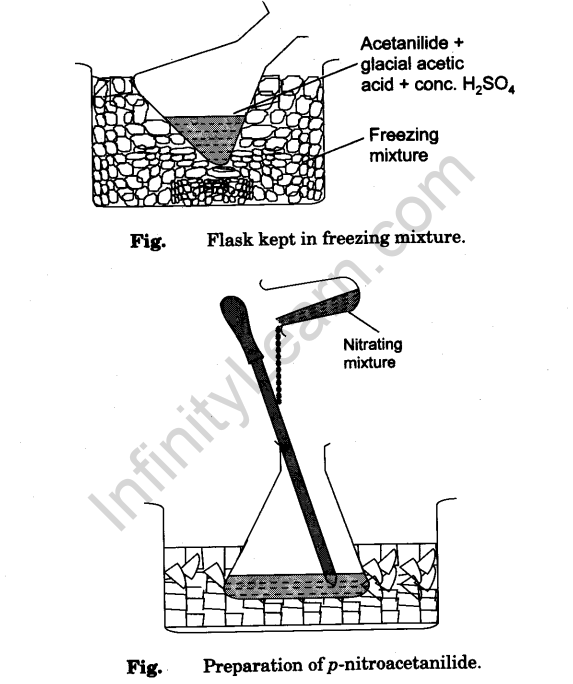
- Add the remaining 8 ml of cone. H2S04 drop by drop (with cooling under tap water) to the conical flask containing acetanilide and glacial acetic acid. Place the conical flask in a freezing mixture (Fig). Stir the contents and wait until the temperature becomes less than 5°C.
- To the cooled contents in the flask add nitrating mixture prepared in step (2) drop by drop with constant stirring. During addition temperature of the mixture should not rise above 10°C. This operation should take about 15 minutes (Fig).
- Remove the conical flask from the freezing mixture and allow it to stand for 30 minutes at room temperature.
- Pour the contents of the flask on the crushed ice taken in a beaker. Stir it and filter the crude product. Wash thoroughly with cold water to remove acid.
- Recrystallisation of p-nitroacetanilide. Dissolve the crude product obtained above in about 20 ml of methylated spirit. Warm to get a clear solution. Filter while hot and cool the filtrate in ice. o-Nitroacetanilide goes in the filtrate while p-nitroacetanilide is obtained as colourless crystals on the filter paper. Wash the solid on the filter paper with cold water. Dry the solid, weigh it and record its yield.
Result
Weight of p-nitroacetanilide is obtained =………g
Melting point of the compound is……….°C
Note: Approximate expected yield is 4 g.
The melting point of p-nitroacetanilide is 214°C.
Precautions
- During addition of nitrating mixture, the temperature of the reaction mixture should not rise above 10°C.
- Addition of fuming nitric acid should be done drop wise.
- Do not inhale the vapours of nitric acid as they are very corrosive in nature. Addition of nitrating mixture may preferably be done in a fume-cupboard.


