Table of Contents
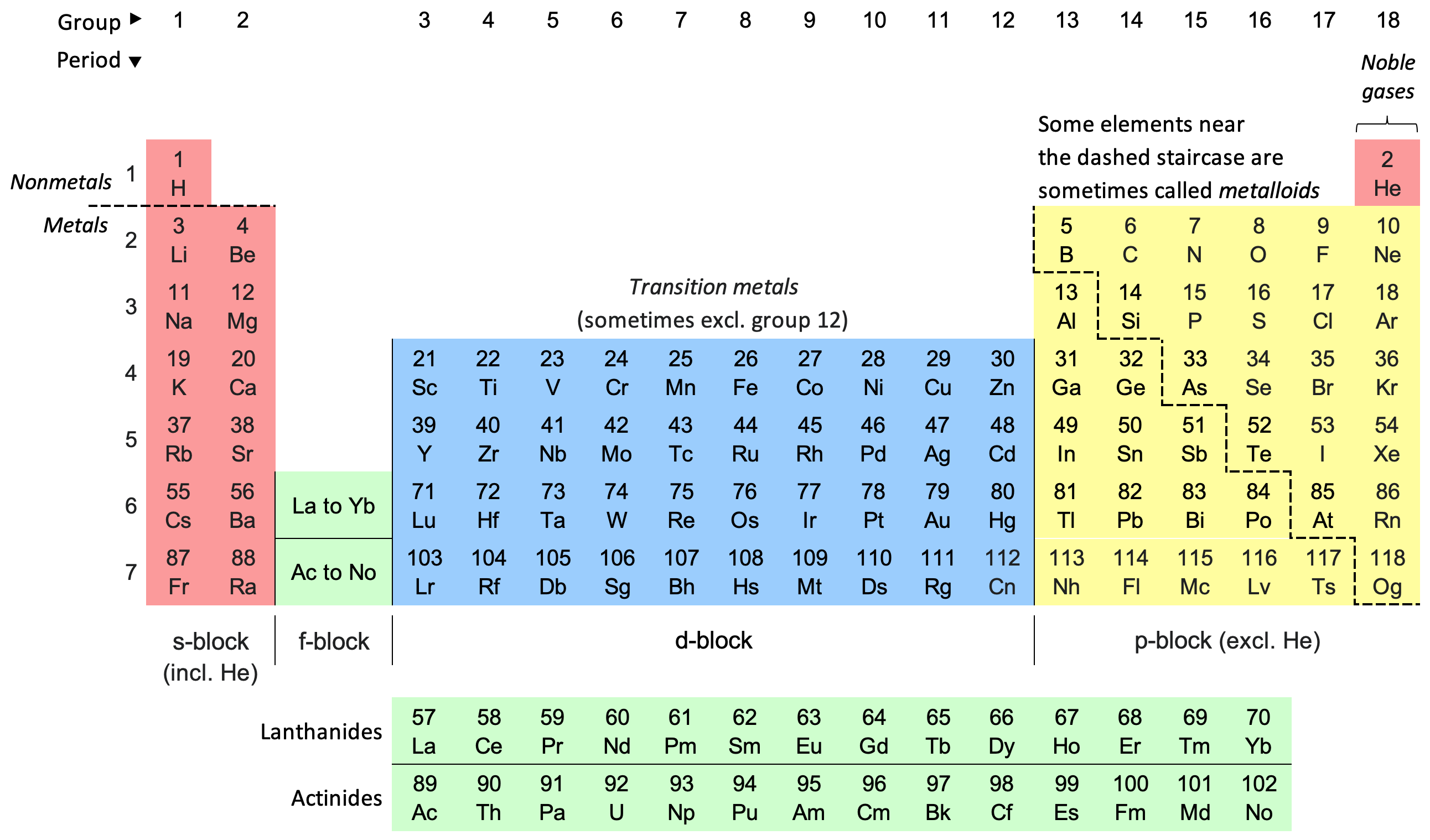
The modern periodic table is a tool used to sort and categorize all the elements we know. It arranges them by their atomic number, electron configuration, and how they behave chemically. Each element is identified by a chemical symbol, with the number above it indicating its atomic number.
The person behind the development of the modern periodic table is Dmitri Mendeleev, often referred to as its father. The table gets its name because it organizes elements in rows (periods) from left to right and columns (groups) from top to bottom.
Modern Periodic Table
The Modern Periodic Table is a structured arrangement of elements, sorted by their atomic numbers in ascending order. It’s also known as the Long Form of the Periodic Table, and it’s based on the modern periodic law.
In this table, you’ll find elements organized in a grid, featuring 18 vertical columns known as Groups and 7 horizontal rows called Periods. Rows are referred to as periods, and columns are called groups.
The first 94 elements in the Modern Periodic Table occur naturally, while elements from 95 to 118 have been created in laboratories or research centers.
Elements within the same group share a similar valence electron configuration, leading to similar chemical properties. Meanwhile, elements in the same period have an increasing number of valence electrons.
The Modern Periodic Table is an improved version of earlier models created by various scientists. Dimitri Mendeleev initially developed the periodic table, and it was later perfected by English physicist Henry Moseley in 1913, addressing the shortcomings of Mendeleev’s table.
Modern Periodic Law
According to Henry Moseley’s Modern Periodic Law, the elements’ physical and chemical characteristics depend on their atomic numbers, not their atomic masses. In the modern periodic table, elements are arranged in order of increasing atomic numbers from left to right in each row. This arrangement reveals that elements with similar traits repeat at regular intervals.
So, why prioritize atomic number over atomic mass? Atomic mass refers to the combined mass of protons and neutrons inside an atom’s nucleus, while atomic number represents the number of protons in the nucleus.
Electrons in the outermost shell, which participate in chemical reactions, are free to move. Hence, an element’s attributes are determined by its atomic number, not its atomic mass.
Modern Periodic Table Periods
The long rows in the modern periodic table are called periods. Each period shows how many energy levels an atom of an element has.
The modern periodic table has 7 periods, numbered 1 to 7 from top to bottom. Each period has a different number of elements. The 1st period has just two elements: Hydrogen and Helium.
The 2nd and 3rd periods contain 8 elements each, while the 4th and 5th periods have 18 elements each. The 6th and 7th periods both consist of 32 elements.
The 7th period introduces four new elements: 113 (Nihonium), 115 (Moscovium), 117 (Tennessine), and 118 (Oganesson).
Additionally, at the bottom of the periodic table, there’s a separate section with 14 elements from the 6th period known as the lanthanoids and 14 elements from the 7th period known as the actinoids.
Modern Periodic Table Groups
In the modern periodic table, the vertical columns are called Groups. Elements within the same group often exhibit similar trends in terms of size, how easily they form ions, and their attraction for electrons. When you move down a group, the size of the atoms generally gets larger.
The long-form periodic table has a total of 18 groups, numbered from 1 to 18. Each group in the table is made up of elements that share a common arrangement of electrons in their outermost shell.
Cause of Periodicity in the Modern Periodic Table
The reason elements show similar traits at regular intervals is due to their outer electron arrangements.
For example, all Group 1 elements share the same outer electron configuration, which is ns1, where “n” stands for the outermost shell’s Principal Quantum Number.
In a similar way, Group 17 elements have the outer electron configuration ns2np5, and because of this, they’re known as halogens and exhibit similar properties.
Group 18 elements have an outer electron arrangement of ns2np6, and these elements possess fully filled orbitals. They’re called Inert gases and are generally unreactive.
Additionally, elements within the same group in the periodic table have akin characteristics because they share a common outer shell electron configuration.
The periodic table is divided into s, p, d, and f blocks based on how valence electrons are positioned within their respective sub-shells.
Modern Periodic Table Classification of Elements
The long form of the periodic table, also known as the modern periodic table, can be divided into several groups of elements, each with its own characteristics:
- Alkali and Alkaline Earth Metals: These are the first two groups on the left side. They are highly reactive, except for hydrogen. The first group has elements with one electron in their outer shell, while the second group has elements with two electrons in their outer shell.
- Transition Metals: These are in the middle of the table and mostly act like typical metals. They span from Group 3 to Group 12. Some special transition metals, called Lanthanides and Actinides, are placed separately at the bottom of the table.
- Metalloids and Non-Metals: Metalloids form a diagonal line on the right side, acting as a bridge between metals and non-metals. They display properties of both.
- Noble Gases: Noble Gases are on the far right in Group 18. They have completely filled outer electron shells, making them unreactive, and they’re also known as inert gases.
The modern periodic table is essential for understanding these elements and their properties, making it possible to study them effectively.
Elements and Atomic Number
The modern periodic table of elements is given in the table below:
| Elements and Atomic Number | ||
| Atomic Number (Z) | Symbol | Element Name |
| 1 | H | Hydrogen |
| 2 | He | Helium |
| 3 | Li | Lithium |
| 4 | Be | Beryllium |
| 5 | B | Boron |
| 6 | C | Carbon |
| 7 | N | Nitrogen |
| 8 | O | Oxygen |
| 9 | F | Fluorine |
| 10 | Ne | Neon |
| 11 | Na | Sodium |
| 12 | Mg | Magnesium |
| 13 | Al | Aluminium |
| 14 | Si | Silicon |
| 15 | P | Phosphorus |
| 16 | S | Sulfur |
| 17 | Cl | Chlorine |
| 18 | Ar | Argon |
| 19 | K | Potassium |
| 20 | Ca | Calcium |
| 21 | Sc | Scandium |
| 22 | Ti | Titanium |
| 23 | V | Vanadium |
| 24 | Cr | Chromium |
| 25 | Mn | Manganese |
| 26 | Fe | Iron |
| 27 | Co | Cobalt |
| 28 | Ni | Nickel |
| 29 | Cu | Copper |
| 30 | Zn | Zinc |
| 31 | Ga | Gallium |
| 32 | Ge | Germanium |
| 33 | As | Arsenic |
| 34 | Se | Selenium |
| 35 | Br | Bromine |
| 36 | Kr | Krypton |
| 37 | Rb | Rubidium |
| 38 | Sr | Strontium |
| 39 | Y | Yttrium |
| 40 | Zr | Zirconium |
| 41 | Nb | Niobium |
| 42 | Mo | Molybdenum |
| 43 | Tc | Technetium |
| 44 | Ru | Ruthenium |
| 45 | Rh | Rhodium |
| 46 | Pd | Palladium |
| 47 | Ag | Silver |
| 48 | Cd | Cadmium |
| 49 | In | Indium |
| 50 | Sn | Tin |
| 51 | Sb | Antimony |
| 52 | Te | Tellurium |
| 53 | I | Iodine |
| 54 | Xe | Xenon |
| 55 | Cs | Caesium |
| 56 | Ba | Barium |
| 57 | La | Lanthanum |
| 58 | Ce | Cerium |
| 59 | Pr | Praseodymium |
| 60 | Nd | Neodymium |
| 61 | Pm | Promethium |
| 62 | Sm | Samarium |
| 63 | Eu | Europium |
| 64 | Gd | Gadolinium |
| 65 | Tb | Terbium |
| 66 | Dy | Dysprosium |
| 67 | Ho | Holmium |
| 68 | Er | Erbium |
| 69 | Tm | Thulium |
| 70 | Yb | Ytterbium |
| 71 | Lu | Lutetium |
| 72 | Hf | Hafnium |
| 73 | Ta | Tantalum |
| 74 | W | Tungsten |
| 75 | Re | Rhenium |
| 76 | Os | Osmium |
| 77 | Ir | Iridium |
| 78 | Pt | Platinum |
| 79 | Au | Gold |
| 80 | Hg | Mercury |
| 81 | Tl | Thallium |
| 82 | Pb | Lead |
| 83 | Bi | Bismuth |
| 84 | Po | Polonium |
| 85 | At | Astatine |
| 86 | Rn | Radon |
| 87 | Fr | Francium |
| 88 | Ra | Radium |
| 89 | Ac | Actinium |
| 90 | Th | Thorium |
| 91 | Pa | Protactinium |
| 92 | U | Uranium |
| 93 | Np | Neptunium |
| 94 | Pu | Plutonium |
| 95 | Am | Americium |
| 96 | Cm | Curium |
| 97 | Bk | Berkelium |
| 98 | Cf | Californium |
| 99 | Es | Einsteinium |
| 100 | Fm | Fermium |
| 101 | Md | Mendelevium |
| 102 | No | Nobelium |
| 103 | Lr | Lawrencium |
| 104 | Rf | Rutherfordium |
| 105 | Db | Dubnium |
| 106 | Sg | Seaborgium |
| 107 | Bh | Bohrium |
| 108 | Hs | Hassium |
| 109 | Mt | Meitnerium |
| 110 | Ds | Darmstadtium |
| 111 | Rg | Roentgenium |
| 112 | Cn | Copernicium |
| 113 | Nh | Nihonium |
| 114 | Fl | Flerovium |
| 115 | Mc | Moscovium |
| 116 | Lv | Livermorium |
| 117 | Ts | Tennessine |
| 118 | Og | Oganesson |
Modern Periodic Table FAQs
What is the Modern Periodic Table?
The Modern Periodic Table is a systematic arrangement of chemical elements based on their atomic numbers, electron configurations, and chemical behaviors. It helps scientists categorize and study the elements effectively.
Who developed the Modern Periodic Table?
The initial periodic table was developed by Dmitri Mendeleev, but it was improved and modernized by Henry Moseley in 1913. Mendeleev is often referred to as the father of the periodic table.
How are elements arranged in the Modern Periodic Table?
Elements are arranged in rows (periods) from left to right and columns (groups) from top to bottom. The arrangement is based on increasing atomic numbers, with similar elements appearing at regular intervals.
What is the significance of atomic numbers in the Modern Periodic Table?
Atomic numbers are crucial because they determine an element's position in the periodic table. Elements are ordered by their atomic numbers, not atomic masses, as per Henry Moseley's Modern Periodic Law.
Why do elements within the same group have similar properties?
Elements within the same group share a common outer electron configuration, which leads to similar chemical properties. For example, all Group 1 elements have one electron in their outermost shell and exhibit similar behavior.









