Table of Contents
Extra Questions Very Short Answer Questions
Question 1.
What are good conductors?
Answer:
The substances that conduct electricity through them are called good conductors.
Question 2.
What are insulators or poor conductors?
Answer:
The substances that do not conduct electricity through them are poor conductors or insulators.
Question 3.
Give four examples of conductors.
Answer:
Copper, iron, aluminium and brass.
Question 4.
Give four examples of insulators.
Answer:
Air, wood, rubber and plastic.
Question 5.
Name two metal objects which have a coating of another metal.
Answer:
Handlebars of bicycles, bathroom taps.
Question 6.
What do we get on electrolysis of acidified water?
Answer:
Hydrogen and oxygen gas.
Question 7.
Is air a bad or good conductor?
Answer:
A bad conductor.
Question 8.
Which metal is plated on handle bars of cycles and rim of wheels?
Answer:
Chromium
Question 9.
What is the full form of LED?
Answer:
Light Emitting Diode.
Question 10.
How do we check the electric current?
Answer:
We check the electric current by using a tester.
Question 11.
Which part of an atom is responsible for flow of current?
Answer:
Electron
Question 12.
Why do most liquids conduct electricity?
Answer:
Due to the presence of ions in them, most liquids conduct electricity.
Question 13.
An LED is more efficient device than a bulb. Why?
Answer:
LED is more efficient because it can glow even when a weak or less current flows through it.
Question 14.
Do lemon juice or vinegar conduct electricity?
Answer:
Yes, they conduct electricity.
Question 15.
How is conductivity of liquids tested?
Answer:
By using a tester.
Question 16.
Is water from taps, handpumps, wells and ponds a good conductor?
Answer:
Yes, water from these sources is a good conductor.
Question 17.
What makes distilled water a good conductor?
Answer:
Salts when mixed with distilled water make it a good conductor.
Question 18.
Why is a layer of zinc coated over iron?
Answer:
To prevent iron from corrosion and rust.
Question 19.
Will the solution of sugar in distilled water conduct electricity?
Answer:
No
Question 20.
Why is tin electroplated on iron to make cans used for storing food?
Answer:
Tin is less reactive than iron. Tin coating prevents food from coming in contact with iron and thus pre¬vents it from getting spoiled or corroded.
Question 21.
Why we use chromium electroplating on taps and bars of bicycle instead of silver and gold?
Answer:
Silver and gold are very expensive comparatively to chromium.
Question 22.
What type of effect of current do the deposits of metal on electrodes show?
Answer:
Chemical effect
Question 23.
What effect of current does electroplating show?
Answer:
Chemical effect
Question 24.
Which effect of current causes the bulb to glow?
Answer:
Heating effect
Question 25.
Which part of the bulb glows?
Answer:
Filament
Question 26.
Name the three effects of electric current.
Answer:
Heating, magnetic and chemical effect.
Question 27.
How can the magnetic effect of current be checked?
Answer:
By using magnetic compass.
Question 28.
What do we see when the compass needle is brought near a wire in which current is flowing?
Answer:
The needle deflects.
Question 29.
What is electroplating?
Answer:
Deposition of thin layer of a metal over other metal by electrolysis is called electroplating.
Chemical Effects of Electric Current Class 8 Extra Questions Short Answer Questions
Question 1.
Define good conductors and poor conductors or insulators.
Answer:
The materials that conduct electricity through them are called good conductors whereas those that do not conduct electricity are called poor conductors or insulators. For example, copper, brass, aluminium, iron, etc., are conductors whereas rubber, plastic, wood, air, etc., are insulators.
Question 2.
How is the conductivity of liquids tested?
Answer:
The free ends of the tester is dipped in the liquid. If the bulb glows, the liquid is said to be a conductor. If not, it is an insulator.
Question 3.
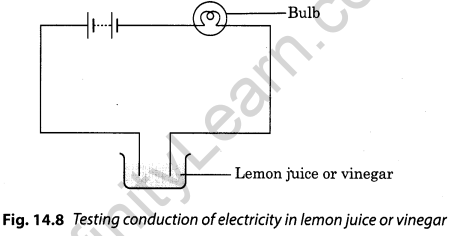
Show with the help of a diagram that lemon juice and vinegar are good conductors of electricity.
Answer:
When the ends of a tester is dipped in lemon juice or vinegar, the bulb glows. This process indicates that lemon juice and vinegar, both, are good conductors of electricity.
Question 4.
What is an LED? Why is it preferred to other type of bulbs?
Answer:
The electric device which is used in the tester instead of bulb is an LED. Its full form is Light Emitting Diode.
It is preferred to other bulbs as it can glow even when weak or less current flows through it.
Question 5.
Explain the conductivity of water.
or
Normal water conducts electricity while the pure or distilled water does not. Explain why?
Answer:
Normal water that we get from sources such as taps, handpumps, wells, ponds, etc., is not pure. It may contain several salts dissolved in it naturally. This water is thus good conductor of electricity. The pure or distilled water is free of salts and is a poor conductor.
Question 6.
Give an example of chemical effect of the electric current.
Answer:
The passage of an electric current through a conducting solution causes chemical reactions as a result, bubbles of a gas are formed, or deposits of metal are seen on electrodes or changes in colour of solution , may occur. These are some of the chemical effects of electric current.
Question 7.
What is electroplating? What are its uses?
Answer:
The process of depositing a layer of any desired metal on another material by means of electricity is called electroplating.
Electroplating is a very useful process. This is used to make objects appear shiny and resistant to scratches. It prevents corrosion.
Question 8.
What happens when electric current is passed through the copper sulphate solution?
Answer:
When electric current is passed through the copper sulphate solution, copper sulphate dissociates into copper and sulphate. The free copper gets drawn to the electrode connected to the negative terminal of the battery and gets deposited on it.
Question 9.
How does a bulb glow in liquid? Explain.
Answer:
When the liquid between the two ends of a tester allows the electric current to pass through it, then the circuit of the tester becomes complete and the current flows in the circuit of liquid makes the bulb glow. But when the liquid does not allow the electric current to pass through it, then the circuit of the tester is incomplete and the bulb does not glow.
Question 10.
What is the magnetic effect of electricity? Explain.
Answer:
When electric current is passed through a coil or wire, it behaves like a magnet. This is known as mag¬netic effect of current. It depends on amount of current passing through the coil or wire.
Question 11.
Why is magnetic compass needed to test the conduction of electric current?
Answer:
Sometimes, when the current passing through a conductor is so small that filament of the bulb does not get heated up and the bulb does not glow. In this case, we need magnetic compass to test the conduction of current.
Question 12.
What happens when an electric current is passed through a cut potato for a considerable time?
Answer:
When an electric current is passed through a cut potato for a considerable time, greenish blue spot is formed around positive electrode. The chemical effect of the electric current is involved in this process.
Question 13.
Why is chromium used for electroplating? Why the objects have chromium plating are not made of chromium itself?
Answer:
Chromium has a shiny look. It does not get corroded and it resists scratches. Chromium is however expensive and it may not be economical to make the whole object out of it. So the object is made from a cheaper metal and only a coating of chromium is done over it.
Question 14.
Which metals, except chromium, are used for electroplating other metals?
Answer:
Jewellery makers electroplate silver and gold on ornaments of less expensive metals.
Tin cans, used for storing food, are made by electroplating tin onto iron. Tin is less reactive than iron. Hence, food is protected from getting spoilt.
Iron used in bridges and automobiles is coated with zinc to protect them from corrosion and formation of rust.
Question 15.
Current does not flow in a circuit if there is a gap between the two wires. Does it indicate that air is a poor conductor of electricity? Does air never conduct electricity? Explain.
Answer:
Air is a poor conductor of electricity if it is dry but in certain cases like during lightning and when air is moist, air may conduct electricity.
Chemical Effects of Electric Current Class 8 Extra Questions Long Answer Questions
Question 1.
On what factors thickness of the electroplated items depend?
Answer:
Thickness of electroplated items depend upon:
- The strength of the current passing through the circuit.
- The concentration of the metal ion in the solution.
- The duration of the time the article has been in the solution.
Question 2.
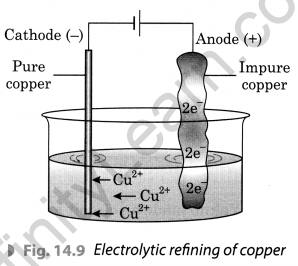
With the help of a suitable diagram, explain electrolytic refining of copper.
Answer:
To purify copper, a thin plate of pure copper and a thick rod of impure copper are used as electrodes in the acidified solution of CuS04. Pure copper is used as cathode and impure copper is used as anode. When electric current is passed through the copper sulphate solution, copper sulphate dissociates into copper and sulphate. The free copper gets drawn to the electrode connected to the negative terminal of the battery and gets deposited on it. From impure copper electrode, an equal amount of copper gets dissolved in the solution. Thus, the loss of copper from solution is restored and the process continues. The impurities are left behind at anode.
Question 3.
Does water conduct electricity? Show with the help of an activity.
or
Show the conductivity of water with the help of an activity.
Answer:
Normal or ordinary water is a good conductor of electricity while distilled water is a bad conductor or insulator. Ordinary water may contain small amount of mineral salts dissolved in it naturally; on the other hand, distilled water is free of salts.
The following activity shows this fact:
About 50 mL of distilled water is taken in a clean and dry beaker. When the tester is dipped into the distilled water, the bulb does not glow which shows that distilled water is a bad conductor of electricity. But when a small amount of common salt is dissolved in distilled water and again tested the bulb glows which shows that distilled water when mixed with salts conduct electricity.
Question 4.
What is electroplating? On which effect of the electric current is it based? Why is it done?
Answer:
The process of depositing or coating a layer of any desired metal on the surface of other material by means of electricity is called electroplating. It is one of the most common applications of chemical effects of electric current.
Electroplating is a very useful process. It is widely used in industry for coating metal objects with a thin layer of a different metal. The layer of metal deposited has some desired property, which the metal of the object lacks. For example, chromium plating is done on many objects to make them shiny and attractive.
Question 5.
What are the advantages and disadvantages of electroplating?
Answer:
Electroplating is a very useful process. It is widely used in industry for coating metal objects with a thin layer of different metal. The advantages and disadvantages of electroplating are:
Advantages:
- It protects the metals from being corroded.
- It prevents the rusting of metals.
- It makes cheap and dull metals shiny and attractive.
- It can make more reactive metals like iron less reactive.
- Chromium coating on metals give lustre to objects.
Disadvantages
- Pollutants from electroplating industries are very harmful. Some chemicals are very lethal for both human and animals.
- It is an expensive process.
Chemical Effects of Electric Current Class 8 Extra Questions Higher Order Thinking Skills
Question 1.
Why electric fires are extinguished with either using CO2 extinguisher or mud but not water?
Answer:
As water is a good conductor of electricity so it can cause electrocution. Hence, water is avoided in extinguishing electric fires.
Question 2.
Why do you think electroplated jewelleries are in demand?
Answer:
Electroplated jewelleries are in demand because firstly, they are as shiny and attractive as real jewelleries. They are light-weighted and cost effective. Secondly, one feels free to wear it because of the growing problem of snatching and theft.
Question 3.
Do distilled water conduct electricity? What will happen if we add sugar to it and then salt to it? Explain.
Answer:
No, distilled water do not conduct electricity. If we add sugar to distilled water, then also it will not conduct electricity because sugar do not dissociates into ions. But on adding salt, it will conduct electricity because aqueous salt solution is a good conductor of electricity.
Question 4.
Suppose you want to deposit silver on an iron spoon using silver nitrate as electrolyte. Which terminal of the battery you should connect the spoon? What material should the other electrode be made of?
Answer:
Silver ion is positively charge, so the spoon must be connected to negative terminal to deposit silver on it. The other electrode should be made of silver.
Question 5.
Why potato turns green on passing current? Around which terminal greenish patch is observed?
Answer:
Potato turns green due to chemical effect of current. Around positive terminal greenish patch in potato is observed.
Chemical Effects of Electric Current Class 8 Extra Questions Value-Based Questions
Question 1.
Yakub made an circuit as shown in the figure. He observed that the bulb did not glow but on bringing a compass needle near it shows deflection.
He was quite confused that if current is flowing through the circuit then why the bulb is not glowing. Meanwhile his friend Sourav arrived and suggested him to add one more cell in the circuit. The bulb, then started glowing.
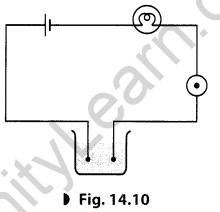
(a) Define a circuit.
(b) What does the deflection of a compass needle shows?
(c) Why the bulb did not glow in the first case but glow in the second case?
(d) What value of Sourav is shown here?
Answer:
(a) Circuit is a closed path through which an electric current flows.
(b) Deflection of compass needle shows that the current is flowing in the circuit. It is magnetic effect of current.
(c) The current flowing through the circuit in first case was too low to make the bulb glow but on adding a cell in the second case makes the bulb glow.
(d) Sourav is an intelligent, helpful, analytical and with scientific aptitude.
Question 2.
While demonstrating an experiment to show whether the given liquid conduct electricity or not to class VIII students, teacher reminded everybody that one should not conduct experiment with the electric supply from the mains or a generator or an inverter. They should use electric cells for the activity.
(a) Do liquids conduct electricity?
(b) Why we should not use electric source from mains generator or an inverter?
(c) What values do we get from this?
Answer:
(a) Yes, liquids which are solutions of acids, bases and salts conduct electricity. Other liquids such as oil, alcohol, sugar solution and pure water do not conduct electricity.
(b) Current flowing from mains, generator or an inverter is very large. So to avoid the chances of elec-trocution and short-circuit we must use cells for experiments.
(c) We get awareness of not using main electric supply and precaution to be followed while doing experiment.
Activities and Projects
Question 1.
Test the conduction of electricity through various fruits and vegetables. Display your re¬sults in a tabular form.
Answer:
Do it yourself.
Question 2.
Repeat Activity 14.7 with a zinc plate in place of the copper plate connected to the negative terminal of the battery. Now replace zinc plate with some other metallic object and again repeat the activity. Which metal gets deposited over which other metal? Discuss your findings with your friends.
Answer:
When zinc plate is taken as negative electrodes copper ions are deposited on zinc plate.
Similarly, the copper ions will be deposited on the plate taken as negative electrode. The process is known as electroplating.
Question 3.
Find out if there is a commercial electroplating unit in your town. What objects are electroplated there and for what purpose? (The process of electroplating in a commercial unit is much more complex than what we did in Activity 14.7). Find out how they dispose off the chemicals they discard.
Answer:
You can find a commercial electroplating unit in your town. Visit the unit and see what objects are electroplated there. Electroplating is a very common and effective method to check corrosion, rusting. The surface of iron metal is coated with aluminium, chromium, nickel, etc. Electroplated items are quite resistant to the attack of external agents like acids, water and corrosion. If the surface of metal is electroplated by zinc, it is called galvanisation. Chemicals or electroplating wastes are hazardous to human health and environment. They are managed according to the Hazardous Wastes Rule and treated accordingly before disposal in the environment.
Question 4.
Imagine that you are an ‘entrepreneur’ and have been provided a loan by a bank to set up a small electroplating unit. What object would you like to electroplate and for what purpose? (Look up the meaning of‘entrepreneur’in a dictionary).
Answer:
You can select the objects of your own choice and interest like electroplating jewellery items with gold and silver, wheel rims of vehicles with nickel, etc. It will make the objects shiny, attractive and durable.
Question 5.
Find out the health concerns associated with chromium electroplating. How are people trying to resolve them?
Answer:
The health concerns associated with chromium electroplating are mainly skin diseases, alteration of genetic material, respiratory problems, weak immune system, upset stomach and ulcers.
In order to resolve these problems chromium are replaced by mild steel and using water insoluble chromium compounds in factories.
Question 6.
You can make a fun pen for yourself. Take a conducting metal plate and spread a moist paste of potassium iodide and starch. Connect the plate to a battery as shown in Fig. 14.7. Now using the free end of the wire, write a few letters on the paste. What do you see?
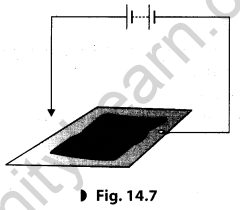
For more information on this topic visit:
electronics.howstuffworks.com/led.htm
Answer:
Do it yourself at home.
I. Multiple Choice Questions (MCQs)
Choose the correct option.
Question 1.
Which of the following is a bad conductor of electricity?
(a) Distilled water
(b) Silver nitrate
(c) Sulphuric acid
(d) Copper sulphate
Question 2.
Which of the following does not conduct electricity?
(a) Sugar solution
(b) Vinegar solution
(c) Lemon juice solution
(d) Caustic soda solution
Question 3.
An electric current can produce
(a) heating effect
(b) chemical effect
(c) magnetic effect
(d) all of these
Question 4.
Pure or distilled water is a
(a) poor conductor
(b) good conductor
(c) both (a) and (b)
(d) none of these
Question 5.
Which of the following is a good conductor?
(a) Brick
(b) Steel
(c) Plastic
(d) Cotton
Question 6.
Polythene is
(a) a conductor
(b) an insulator
(c) both (a) and (b)
(d) none of these
Question 7.
Electroplating is based on
(a) heating effect of electricity
(b) chemical effect of electricity
(c) physical effect of electricity
(d) magnetic effect of electricity
Question 8.
Copper is
(a) a good conductor
(b) an insulator
(c) both (a) and (b)
(d) none of these
Question 9.
Waste from an electroplating factory must be disposed off
(a) in the nearby river
(b) in the nearby pond
(c) in the nearby cornfield
(d) according to the disposal guidelines of Waste Management Bodies
Question 10.
An electrolyte is
(a) a metal
(b) a liquid that conducts current
(c) a non-metal
(d) none of these
Question 11.
Flow of electron is called
(a) electrolyte
(b) electroplating
(c) electrodes
(d) electric current
Question 12.
Which is not a non-electrolyte?
(a) Ethyl alcohol
(b) Sodium chloride
(c) Urea
(d) Sodium solution
Question 13.
An electric lamp glows due to
(a) heating effect
(b) magnetic effect
(c) chemical effect
(d) physical effect
Question 14.
Electroplating prevents
(a) corrosion
(b) passing of current
(c) dissociation
(d) shining
Question 15.
Which of the following is not used for electroplating metal articles?
(a) Nickel
(b) Silver
(c) Chromium
(d) Sodium
Question 16.
Iron objects can be protected by electroplating them with
(a) chromium
(b) nickel
(c) zinc
(d) all of these
Answer:
1. (a)
2. (a)
3. (d)
4. (a)
5. (b)
6. (b)
7. (b)
8. (a)
9. (d)
10. (b)
11. (d)
12. (b)
13. (a)
14. (a)
15. (d)
16. (d)
II. Fill in the Blanks
Fill in the blanks with suitable word/s.
1. Substances that conduct electricity are called ___________.
2. Substances that do not conduct electricity are called ___________.
3. A cation has ___________ charge.
4. Some liquids are ___________ conductors of electricity and some are ___________ conductors of electricity.
5. Distilled water is an ___________.
6. Distilled water when mixed with salts becomes a ___________ conductor of electricity.
7. Light emitting diodes (LED) glow even when a ___________ electric current flows through it.
8. The passage of an electric current through a conducting solution causes ___________.
9. Change in colour is an example of the ___________ effect of current.
10. There are ___________ wires attached to an LED.
11. In an LED, the longer lead is attached to the ___________ terminal of the battery and the shorter lead to the ___________ terminal.
12. Chromium has a ___________ appearance.
13. Distilled water is made by removing all ___________.
14. Iron tends to ___________ and ___________.
15. A coating of ___________ is deposited on iron to protect it from corrosion and formation of rust.
16. An electric lamp glows due to ___________ effect of electric current.
17. Electrodes are ___________.
18. The deflection in ___________ shows that current is passing.
19. ___________does not corrode easily.
20. An electrolyte is a ___________.
Answer:
1 conductors
2. insulator
3. positive
4. good, poor
5. insulator
6. good
7. weak
8. chemical reaction
9. chemical
10. two
11. positive, negative
12. shiny
13. impurities
14. corrode, rust
15. zinc
16. heating
17. conductors
18. magnetic compass
19. Chromium
20. liquid
III. Match the following
Match the items given in column I suitably with those given in column II.
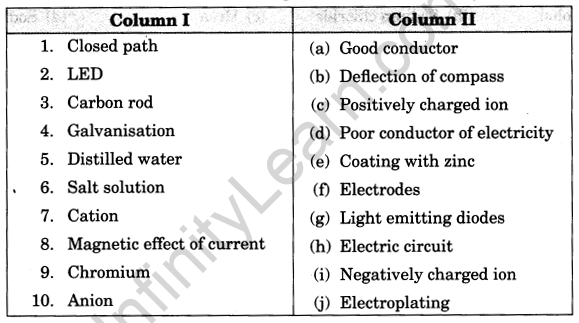
Answer:
1. (h)
2. (g)
3. (f)
4. (e)
5. (d)
6. (a)
7. (c)
8. (b)
9. (j)
10. (i)
IV. True or False
State whether the given statements are true or false.
1. Rubber is a good conductor of electricity.
2. Plastics are poor conductor of electricity.
3. All liquids conduct electricity.
4. Distilled water is free of salt.
5. Pure water conducts electricity.
6. Most liquids that conducts electricity are solutions of acids, bases and salts.
7. Electroplating is based on magnetic effect of electricity.
8. Small amount of some mineral salts are naturally present in water.
9. Chromium is carcinogenic.
10. An electric bulb glows due to chemical effect of electricity.
11. Distilled water when mixed with salt conducts electricity.
12. LED is an electric bulb which is used in a tester.
13. Deflection in compass needle is due to magnetic effect of current.
14. When electric current is passed through the copper sulphate solution, copper and sulphate ions are dissociated.
15. In an LED bulb, the shorter lead is connected to the positive terminal of the battery.
16. Electric current produces a magnetic effect.
17. Some liquids are good conductors of electricity and some are poor conductors of electricity.
18. Chromium has a shiny appearance.
19. Jewellery makers electroplate silver and gold on expensive metals.
20. Electroplating wastes are useful to human health and environment.
Answer:
1. False
2. True
3. False
4. True
5. False
6. True
7. False
8. True
9. True
10. False
11. True
12. True
13. True
14. True
15. False
16. True
17. True
18. True
19. False
20. False







