Table of Contents
Introduction
Ammonium nitrate is a chemical compound with the formula NH4NO3. It is a white crystalline solid that is highly soluble in water. Ammonium nitrate is commonly used as a fertilizer in agriculture due to its high nitrogen content, which is essential for plant growth. It provides a readily available source of nitrogen to plants, promoting their development and improving crop yields.
In addition to its use as a fertilizer, ammonium nitrate is also employed in the production of explosives, particularly in mining and construction industries. It is a key component of ANFO (ammonium nitrate/fuel oil) explosives, which are widely used for blasting operations due to their stability and effectiveness.
Chemical and Structural Formula of Ammonium Nitrate
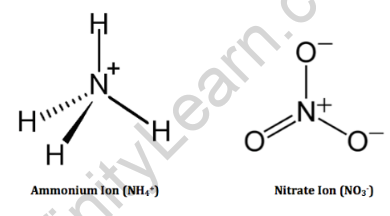
- The chemical formula for ammonium nitrate is NH4NO3. It consists of the ammonium ion (NH4+) and the nitrate ion (NO3–).
- The ammonium ion is a positively charged polyatomic ion composed of one nitrogen atom bonded to four hydrogen atoms.
- The nitrate ion is a negatively charged polyatomic ion composed of one nitrogen atom bonded to three oxygen atoms.
- When these ions combine, they form ammonium nitrate, which is a white crystalline solid commonly used as a fertilizer and an explosive material.
The structural formula is as follows:
Physical Properties of Ammonium Nitrate
- Appearance: Ammonium nitrate is a white crystalline solid. It is typically found in the form of small crystals or granules.
- Solubility: Ammonium nitrate is highly soluble in water. It dissolves readily, forming a clear solution.
- Melting Point: The melting point of ammonium nitrate is approximately 169.6 degrees Celsius (337.3 degrees Fahrenheit). At this temperature, it undergoes a phase change from a solid to a liquid.
- Density: The density of solid ammonium nitrate is around 1.72 grams per cubic centimeter. The density of the aqueous solution varies with concentration.
- Odor: Ammonium nitrate is odorless.
- Hygroscopicity: Ammonium nitrate has hygroscopic properties, meaning it has the ability to absorb moisture from the surrounding atmosphere. This characteristic can cause it to become damp or dissolve in humid conditions.
Chemical Properties of Ammonium Nitrate
- Decomposition: Ammonium nitrate is a highly reactive compound that undergoes decomposition when heated. The decomposition reaction produces nitrogen gas (N2), water (H2O), and oxygen gas (O2). This decomposition is exothermic, meaning it releases heat.
- Oxidizing Agent: Ammonium nitrate is an effective oxidizing agent. It provides a source of oxygen that can support combustion when in contact with flammable materials. This property makes it useful in applications such as fertilizers and explosives.
- pH: When dissolved in water, ammonium nitrate dissociates into ammonium ions (NH4+) and nitrate ions (NO3–). The presence of the ammonium ion makes the solution slightly acidic.
- Hygroscopicity: Ammonium nitrate has hygroscopic properties, meaning it has the ability to absorb moisture from the air. This can lead to caking or clumping of the material, especially in humid conditions.
- Stability: Ammonium nitrate is stable under normal conditions. However, it can become more sensitive to decomposition when exposed to high temperatures, shocks, or contamination with certain substances. Proper storage and handling are important to prevent accidental detonation.
- Reaction with Metals: Ammonium nitrate can react with certain metals, particularly reactive metals like aluminum. The reaction can be highly exothermic and may result in the release of flammable gases or even an explosion.
Also Check: Zinc Nitrate Formula
Conclusion
In conclusion, ammonium nitrate is a chemical compound with the formula NH4NO3. It is widely used as a fertilizer in agriculture due to its high nitrogen content, which promotes plant growth. However, ammonium nitrate also possesses explosive properties and has been involved in several accidents and incidents.
Its potential for misuse and the risks associated with mishandling have led to strict regulations and controls on its storage, transportation, and sale. These measures aim to ensure public safety and prevent unauthorized or unsafe use of ammonium nitrate. Understanding its properties and following appropriate safety protocols are crucial in handling ammonium nitrate to mitigate potential risks and promote responsible use.
Solved Examples on Ammonium Nitrate Formula
Example 1: Calculate the mass of ammonium nitrate needed to prepare 500 mL of a 0.2 M ammonium nitrate solution.
Solution: Step 1: Determine the molar mass of ammonium nitrate (NH4NO3).
Molar mass of NH4NO3 = (1 x Atomic mass of N) + (4 x Atomic mass of H) + (3 x Atomic mass of O)
= (1 x 14.01 g/mol) + (4 x 1.01 g/mol) + (3 x 16.00 g/mol)
= 80.05 g/mol
Step 2: Calculate the moles of ammonium nitrate needed.
Moles = Molarity x Volume
= 0.2 mol/L x 0.5 L = 0.1 mol
Step 3: Calculate the mass of ammonium nitrate. Mass = Moles x Molar mass = 0.1 mol x 80.05 g/mol = 8.005 g
Therefore, 8.005 grams of ammonium nitrate are needed to prepare 500 mL of a 0.2 M ammonium nitrate solution.
Example 2: Ammonium nitrate decomposes upon heating to produce nitrogen gas, water vapor, and oxygen gas. Calculate the volume of oxygen gas produced when 50 grams of ammonium nitrate decomposes.
Solution: Step 1: Determine the moles of ammonium nitrate.
Moles = Mass / Molar mass
= 50 g / 80.05 g/mol (molar mass of NH4NO3)
= 0.624 moles
Step 2: Use the balanced chemical equation to determine the stoichiometry.
According to the balanced equation:
2NH4NO3(s) → 2N2(g) + 4H2O(g) + O2(g)
1 mole of NH4NO3 produces 0.5 moles of O2 gas.
Step 3: Calculate the moles of oxygen gas produced.
Moles of O2 = 0.5 x Moles of NH4NO3
= 0.5 x 0.624 moles
= 0.312 moles
Step 4: Convert moles to volume using the ideal gas law.
Volume = Moles x Molar volume (at standard temperature and pressure)
= 0.312 moles x 22.4 L/mol
= 6.9888 L
Therefore, approximately 6.99 liters of oxygen gas is produced when 50 grams of ammonium nitrate decomposes.
Frequently Asked Questions on Ammonium Nitrate Formula
What is the most common use for ammonium nitrate?
The most common use for ammonium nitrate is as a fertilizer. Due to its high nitrogen content, ammonium nitrate provides essential nutrients for plant growth, making it a widely used fertilizer in agriculture. It is particularly effective for crops that require a significant nitrogen supply, such as corn, wheat, and potatoes.
Why ammonium nitrate is banned?
Ammonium nitrate is not universally banned, but its sale, handling, and storage are regulated in many countries due to safety concerns. The primary reason for these regulations is the potential for misuse of ammonium nitrate as an explosive material.
Why is ammonium nitrate acidic?
Ammonium nitrate is not acidic; it is actually a neutral compound. The term ammonium nitrate refers to a salt that is formed from the reaction between the weak base ammonia and the strong acid nitric acid. When ammonia reacts with nitric acid, the ammonia molecule acts as a base, accepting a hydrogen ion from the nitric acid molecule to form the ammonium ion. The remaining part of the nitric acid molecule combines with the negatively charged nitrate ion to form the nitrate component of ammonium nitrate. The resulting compound, ammonium nitrate, contains both the ammonium ion and the nitrate ion. These ions do not have a net excess of hydrogen ions or hydroxide ions, and therefore, the compound is considered neutral.
What is the greatest hazard of ammonium nitrate?
The greatest hazard of ammonium nitrate is its potential to explode under certain conditions. Ammonium nitrate is classified as an explosive substance, particularly when it is in a concentrated form and exposed to a source of ignition or heat. If ammonium nitrate undergoes rapid decomposition or ignition, it can lead to a highly destructive and powerful explosion. The primary factor that contributes to the explosive hazard of ammonium nitrate is its ability to release a large amount of oxygen when it decomposes. This oxygen can support the rapid combustion of other materials, such as fuels or organic substances, leading to a violent reaction and explosion.









