Table of Contents
Introduction
Methanol, also known as methyl alcohol or wood alcohol, is a chemical compound with the formula CH3OH. It is the simplest alcohol, consisting of a methyl group (CH3) bonded to a hydroxyl group (OH). Methanol is a colourless, volatile liquid that is highly flammable. It has a distinctive odour and is miscible in water.
Structural Formula of Methanol
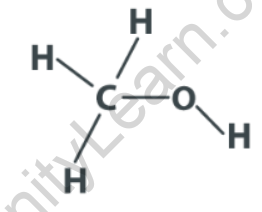
In the structural formula, the central carbon atom (C) is bonded to three hydrogen atoms (H) and one oxygen atom (O). The oxygen atom is also bonded to the carbon atom through a single bond, and it has two lone pairs of electrons.
Uses of Methanol
- Fuel: Methanol is used as an alternative fuel source and can be blended with gasoline or used as a standalone fuel in vehicles. It is commonly used in racing cars and as a fuel additive in certain countries.
- Solvent: Methanol is a versatile solvent and is widely used in industrial processes. It is used as a solvent for paints, varnishes, dyes, and inks. It is also used in the production of various chemicals and pharmaceuticals.
- Chemical Intermediary: Methanol serves as a building block for the production of other chemicals. It is used in the synthesis of formaldehyde, acetic acid, and other organic compounds. These chemicals find applications in the production of plastics, textiles, resins, and adhesives.
- Antifreeze: Methanol is commonly used as an antifreeze agent in automotive cooling systems and windshield washer fluid. It helps prevent freezing and maintains the fluidity of liquids in low-temperature conditions.
- Renewable Energy: Methanol can be produced from renewable sources such as biomass and serves as a potential source of clean energy. It can be used in fuel cells and as a storage medium for hydrogen.
- Laboratory and Analytical Applications: Methanol is commonly used in laboratories as a solvent for chemical reactions and for cleaning glassware. It is also used in analytical techniques such as gas chromatography.
Physical Properties of Methanol
- State: Methanol is a colorless liquid at room temperature.
- Odour: It has a characteristic sweet odour.
- Density: The density of methanol is approximately 0.7918 g/cm³.
- Boiling Point: Methanol has a boiling point of 64.7°C (148.5°F).
- Melting Point: The melting point of methanol is -97.6°C (-143.7°F).
- Solubility: Methanol is highly soluble in water, as it can form hydrogen bonds with water molecules.
- Vapour Pressure: Methanol has a relatively high vapor pressure at room temperature.
- Flammability: Methanol is highly flammable and can form explosive mixtures with air.
- Toxicity: Methanol is toxic and can cause severe health effects if ingested, inhaled, or absorbed through the skin.
Chemical Properties of Methanol
- Reactivity: Methanol is a highly reactive compound. It can undergo various chemical reactions, such as oxidation, esterification, and condensation reactions.
- Combustibility: Methanol is highly flammable and can easily ignite. It burns in the presence of oxygen to produce carbon dioxide and water.
- Acid-Base Properties: Methanol can act as a weak acid in certain reactions, donating a proton (H+) to a base. It can also act as a weak base by accepting a proton from an acid.
- Solvent Properties: Methanol is a good solvent and can dissolve many polar and nonpolar compounds. It is commonly used as a solvent in various chemical processes and industries.
- Hydrogen Bonding: Methanol can form hydrogen bonds with other molecules, particularly with water molecules. This property contributes to its high solubility in water.
- Oxidation: Methanol can be oxidized to formaldehyde (CH2O) and further to formic acid (HCOOH) or carbon dioxide (CO2) under certain conditions.
- Chemical Reactions: Methanol can undergo reactions such as esterification, transesterification, dehydration, and hydrolysis, which are commonly employed in organic synthesis and industrial processes.
- Toxicity: Methanol is toxic and can cause serious health effects, including central nervous system depression, blindness, and even death if ingested or absorbed in large quantities.
Conclusion
In conclusion, methanol, with the chemical formula CH3OH, is a vital compound with numerous applications in various industries. It is commonly used as a solvent, fuel, and chemical intermediate. Methanol is also utilized in the production of formaldehyde, acetic acid, and other chemicals. However, it is important to recognize the potential hazards associated with methanol, as it is highly flammable and toxic. Proper handling, storage, and safety measures are essential when working with methanol to ensure its safe and effective use.
Solved Examples on Methanol Formula
Example 1: Calculate the molar mass of methanol (CH3OH).
Solution:
The molar mass of carbon (C) = 12.01 g/mol
The molar mass of hydrogen (H) = 1.008 g/mol
The molar mass of oxygen (O) = 16.00 g/mol
Molar mass of methanol (CH3OH) = (1 × molar mass of C) + (4 × molar mass of H) + (1 × molar mass of O) = (1 × 12.01) + (4 × 1.008) + (1 × 16.00)
= 32.04 g/mol
Therefore, the molar mass of methanol is 32.04 g/mol.
Example 2: What volume of methanol (CH3OH) is needed to completely react with 10.0 g of oxygen gas (O2) according to the following balanced equation?
2CH3OH + 3O2 → 2CO2 + 4H2O
Solution:
Step 1: Convert the mass of oxygen gas to moles.
Molar mass of O2 = 2 × 16.00 g/mol = 32.00 g/mol
Number of moles of O2 = 10.0 g / 32.00 g/mol = 0.3125 mol
Step 2: Use the stoichiometry of the balanced equation to determine the ratio of moles between O2 and CH3OH.
According to the balanced equation, 3 moles of O2 react with 2 moles of CH3OH.
Step 3: Calculate the moles of CH3OH required.
Number of moles of CH3OH = (0.3125 mol O2) × (2 mol CH3OH / 3 mol O2) = 0.2083 mol
Step 4: Convert the moles of CH3OH to volume using the molar volume at standard conditions (STP).
Molar volume of gas at STP = 22.4 L/mol Volume of CH3OH
= 0.2083 mol × 22.4 L/mol = 4.6592 L
Therefore, 4.6592 liters of methanol (CH3OH) are needed to completely react with 10.0 grams of oxygen gas (O2).
Frequently Asked Questions on Methanol
1: What is the functional formula of methanol?
Answer: The functional formula of methanol is CH3OH. It consists of a methyl group (-CH3) attached to a hydroxyl group (-OH). The hydroxyl group makes methanol a primary alcohol, which means the hydroxyl group is attached to a carbon atom that is only bonded to one other carbon atom.
2: What is the Colour of methanol?
Answer: Methanol is a colorless liquid. It does not have any inherent color, and it appears transparent to the naked eye.
3: How is methanol produced formula?
Answer: Methanol is primarily produced through the catalytic reaction of carbon monoxide (CO) and hydrogen (H2) in the presence of a catalyst. This process is known as the synthesis gas reaction or the steam reforming of natural gas. The overall reaction can be represented by the following equation:
CO + 2H2 -> CH3OH
In this reaction, carbon monoxide and hydrogen react to form methanol. The process is typically carried out under high pressure and temperature conditions, with the aid of a catalyst such as copper or zinc.
4: What is the old name of methanol?
Answer: Methanol, also known as methyl alcohol, carbinol, wood alcohol, wood naphtha or wood spirits, is a chemical compound with chemical formula CH3OH (often abbreviated MeOH).
5: Who is the largest importer of methanol in India?
Answer: India imports most of its Methanol from Iran, Saudi Arabia and Germany and is the largest importer of Methanol in the World. The top 3 importers of Methanol are India with 87,836 shipments followed by Vietnam with 22,700 and United States at the 3rd spot with 8,935 shipments.
6: How is methanol toxic to humans?
Answer: Methanol is toxic to humans due to its metabolic breakdown in the body. When ingested or absorbed, methanol is metabolized in the liver into formaldehyde and formic acid, which are highly toxic substances. These metabolites can cause severe damage to various organs, particularly the optic nerve, central nervous system, and kidneys. Methanol poisoning can lead to symptoms such as dizziness, headache, nausea, vomiting, abdominal pain, visual disturbances, and in severe cases, coma or death. It is crucial to exercise caution and adhere to safety guidelines when handling methanol to prevent accidental exposure and poisoning.
7: Is methanol in drinking alcohol?
Answer: Methanol is not intentionally present in drinking alcohol. However, it can be produced as a byproduct of improper distillation or fermentation processes, particularly in homemade or illicitly produced alcoholic beverages. Contamination with methanol in such cases can be dangerous as methanol is highly toxic when ingested. Commercially produced alcoholic beverages, including those that are properly regulated and tested, should not contain significant levels of methanol. It is important to consume alcohol from reputable sources and to exercise caution when consuming homemade or unregulated alcoholic beverages.
8: Why don’t we drink methanol?
Answer: We don’t drink methanol because it is highly toxic to humans. Methanol is metabolized in the body into formaldehyde and formic acid, which can cause severe damage to various organs, including the liver, kidneys, and central nervous system. Even small amounts of methanol ingestion can lead to serious health complications, including blindness and death. Methanol is a dangerous substance and should never be consumed as a beverage.









