Table of Contents
Introduction to Aluminium Phosphate Formula
Aluminium phosphate is a chemical compound with the formula AlPO4. It is an inorganic salt that consists of aluminium cations (Al3+) and phosphate anions (PO43-).
Aluminium phosphate is a white crystalline solid that is insoluble in water. It has various applications in different fields. Aluminium phosphate finds use as a catalyst in organic synthesis reactions, where it can facilitate chemical transformations. It is also employed as a binding agent in ceramics and refractory materials due to its high temperature stability. In addition, aluminium phosphate is utilized in the production of dental cements, where it acts as a filler material to enhance mechanical properties.
Structural Formula of Aluminium Phosphate Formula
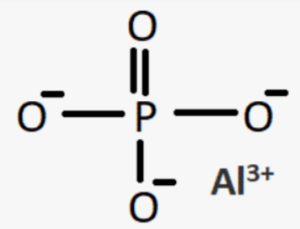
In this structure, the aluminium ion (Al3+) is bonded to four oxygen atoms (O) through ionic bonds. The phosphate ion (PO43-) consists of one phosphorus atom (P) bonded to four oxygen atoms (O) through covalent bonds. The structure forms a three-dimensional network where the aluminium ions and phosphate ions are connected by oxygen atoms. This arrangement results in the formation of a crystalline solid with a repeating unit of AlPO4.
Uses of Aluminium Phosphate
- Pharmaceutical Industry: Aluminium phosphate is commonly used in the pharmaceutical industry as an excipient in the formulation of tablets and capsules. It can serve as a binder, disintegrant, and filler, helping to improve the cohesion and stability of the final pharmaceutical product.
- Vaccine Adjuvant: Aluminium phosphate is used as an adjuvant in vaccines to enhance the immune response. It helps stimulate a stronger and more effective immune reaction to the antigen present in the vaccine.
- Flame Retardant: Aluminium phosphate is utilized as a flame retardant in various materials, including plastics, textiles, and coatings. It acts by releasing water vapor when exposed to heat, which helps cool the material and suppress the spread of flames.
- Dental Cements: Aluminium phosphate is used in the formulation of dental cements. It helps provide the required strength and stability for dental restorations such as fillings, crowns, and bridges.
- Ceramics Industry: Aluminium phosphate is used in the production of ceramics, including pottery, tiles, and refractory materials. It helps improve the mechanical strength, thermal stability, and resistance to chemical corrosion of these ceramic products.
- Water Treatment: Aluminium phosphate is employed in water treatment processes to remove impurities and enhance the clarity of water. It can assist in the coagulation and flocculation of suspended particles, improving the efficiency of water purification.
Physical Properties of Aluminium Phosphate Formula
- State: Aluminium phosphate exists as a solid at room temperature and pressure.
- Color: It is typically a white, crystalline substance. However, it can also appear as a colorless or slightly off-white powder.
- Melting Point: Aluminium phosphate has a relatively high melting point, typically around 1,800 to 1,900 degrees Celsius (3,272 to 3,452 degrees Fahrenheit).
- Density: The density of aluminium phosphate varies depending on the specific crystalline form, but it generally falls within the range of 2.6 to 2.8 grams per cubic centimeter (g/cm³).
- Solubility: Aluminium phosphate is insoluble in water, which means it does not readily dissolve in water to form a homogeneous solution. It is also sparingly soluble in other solvents.
- Stability: Aluminium phosphate exhibits good thermal stability, retaining its structural integrity at high temperatures.
- Crystal Structure: Aluminium phosphate can have various crystal structures, including both crystalline and amorphous forms.
Chemical Properties of Aluminium Phosphate Formula
- Acidic Nature: Aluminium phosphate is amphoteric, meaning it can act as both an acid and a base. It can react with both acids and bases to form salts.
- Insolubility: Aluminium phosphate is insoluble in water and most common solvents. It does not readily dissolve in these substances, which contributes to its stability and use as a solid material.
- Stability: Aluminium phosphate is chemically stable under normal conditions. It maintains its structural integrity and does not undergo significant decomposition or reaction with common chemicals.
- Catalytic Activity: Aluminium phosphate can function as a catalyst in various chemical reactions, including organic synthesis. Its catalytic properties are attributed to its surface acidity and ability to interact with reactant molecules.
- Complex Formation: Aluminium phosphate can form complexes with certain organic and inorganic compounds. These complexes can exhibit unique properties and have applications in fields such as drug delivery and chemical separations.
- Acid-Base Reactions: As an amphoteric compound, aluminium phosphate can undergo acid-base reactions. It can react with strong acids or bases to form salts, where aluminium ions and phosphate ions exchange partners.
- Binding and Adsorption: Aluminium phosphate has the ability to bind or adsorb certain substances due to its surface properties. This property is utilized in applications such as ceramics, where it acts as a binding agent, and in pharmaceutical formulations, where it can adsorb or stabilize active ingredients.
Conclusion
In conclusion, the formula for ammonium phosphate is (NH4)3PO4. Ammonium phosphate is a compound commonly used in fertilizers and as a source of phosphorous and nitrogen for plants. It provides essential nutrients for plant growth and is particularly beneficial for promoting root development, flowering, and fruiting. Ammonium phosphate is also used in some fire extinguishers due to its fire-retardant properties. However, it should be handled with care as it can be corrosive and may cause skin and eye irritation. Proper usage and adherence to safety guidelines are important when working with ammonium phosphate.
Solved Examples on Aluminium Phosphate Formula
Example 1: Calculate the molar mass of aluminium phosphate (AlPO4).
Solution: To calculate the molar mass of aluminium phosphate, we need to determine the atomic masses of the elements involved and sum them up according to their respective stoichiometric ratios.
The atomic mass of aluminium (Al) is approximately 26.98 g/mol.
The atomic mass of phosphorus (P) is approximately 30.97 g/mol.
The atomic mass of oxygen (O) is approximately 16.00 g/mol.
In one molecule of aluminium phosphate (AlPO4), we have: 1 atom of aluminium (Al) 1 atom of phosphorus (P) 4 atoms of oxygen (O).
So, the molar mass of aluminium phosphate is calculated as follows:
Molar mass = (1 × atomic mass of Al) + (1 × atomic mass of P) + (4 × atomic mass of O) = (1 × 26.98 g/mol) + (1 × 30.97 g/mol) + (4 × 16.00 g/mol)
= 26.98 g/mol + 30.97 g/mol + 64.00 g/mol
= 121.95 g/mol
Therefore, the molar mass of aluminium phosphate is approximately 121.95 g/mol.
Example 2: A 0.50 mol/L solution of aluminium phosphate is prepared by dissolving a certain amount of AlPO4 in water. Calculate the number of moles of aluminium phosphate present in 250 mL of this solution.
Solution:
Given: Molarity (M) = 0.50 mol/L
Volume (V) = 250 mL = 0.250 L
We can use the formula for calculating the number of moles:
Number of moles (n) = Molarity (M) × Volume (V)
Plugging in the values:
Number of moles (n) = 0.50 mol/L × 0.250 L = 0.125 mol
Therefore, there are 0.125 moles of aluminium phosphate present in 250 mL of the solution.
Frequently Asked Questions on Aluminium Phosphate Formula
What is Aluminium phosphate used for?
Aluminium phosphate is used in making medicines and as a food additive.
Is Aluminum Phosphate harmful to humans?
Aluminum phosphate in small amounts is generally safe, but in large quantities, it can be harmful.
What is the formula for aluminum phosphate?
The formula for aluminum phosphate is AlPO4.
Is Aluminium phosphate an acid base or salt?
Aluminium phosphate is considered a salt.
How do you write Aluminium phosphate?
You write it as Aluminium phosphate or Aluminum phosphate.
What is the chemical formula for aluminum phosphate?
The chemical formula for aluminum phosphate is AlPO4.
What is another name for aluminum phosphate?
Another name for aluminum phosphate is aluminum orthophosphate.
Is Aluminium phosphate a salt?
Yes, aluminium phosphate is a type of salt.
What is the short form of Aluminium phosphate?
The short form of aluminium phosphate is AlPO4.









