Table of Contents
The Earth’s ecosystems are intricate and dynamic, with the constant flow of matter and energy. Biogeochemical cycles are at the heart of this intricate dance, allowing elements and compounds to circulate and be reused, maintaining the delicate balance of life. In this article, we delve into the fascinating world of biogeochemical cycles, focusing on the carbon, nitrogen, and phosphorus cycles, while also exploring the concept and types of biogeochemical cycles.
Biogeochemical Cycle
A biogeochemical cycle is a natural process that describes the movement and transformation of essential chemical elements and compounds between living organisms (bio), the Earth’s surface (geo), and the atmosphere. These cycles involve the uptake, utilization, and release of elements by living organisms and their return to the environment, creating a continuous loop. Biogeochemical cycles are essential for maintaining the availability of these elements for life on Earth.
Various Types of Biogeochemical Cycles
Sedimentary Cycles
Sedimentary cycles involve the slow movement of elements between the Earth’s surface and sediments or rock layers. Examples include the phosphorus cycle, where phosphorus is released from weathered rocks and gradually transported to aquatic ecosystems, where it becomes available for uptake by plants.
Gaseous Cycles
Gaseous cycles involve the exchange of elements in a gaseous state between the atmosphere and living organisms. The carbon and nitrogen cycles are prominent examples. In gaseous cycles, elements may enter the atmosphere as gases (e.g., carbon dioxide in the carbon cycle) and be incorporated into organisms through processes like photosynthesis.
The Carbon Cycle: Nature’s Breath
Carbon Sources
The carbon cycle involves various sources, including the atmosphere (carbon dioxide), fossil fuels, plants, and organic matter in soil. Carbon enters the atmosphere through respiration, combustion, and volcanic activity.
Carbon Sinks
Carbon is stored in several sinks, such as the oceans, forests, and soil. These sinks absorb and store carbon dioxide, helping regulate atmospheric carbon levels.
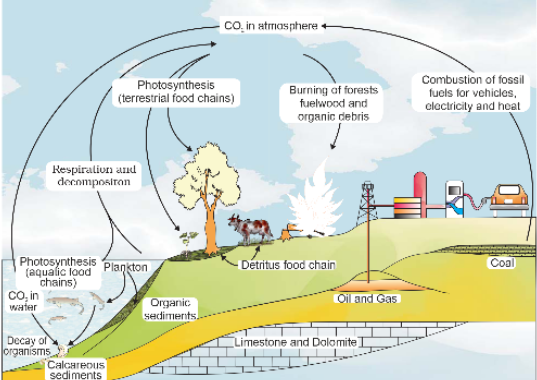
The Carbon Cycle in Action
When examining the composition of living organisms, it becomes evident that carbon constitutes a substantial portion, accounting for 49 percent of the dry weight of these organisms, second only to water in abundance. Surprisingly, when we consider the total quantity of global carbon, a whopping 71 percent of it is dissolved in the world’s oceans. This vast oceanic reservoir plays a crucial role in regulating the amount of carbon dioxide present in the Earth’s atmosphere. It may surprise you to learn that the atmosphere itself contains only a mere 1 percent of the Earth’s total carbon.
Fossil fuels represent another significant reservoir of carbon, integral to the planet’s carbon cycling processes. These cycles involve the exchange of carbon between the atmosphere, the oceans, and the realm of both living and deceased organisms. To provide a sense of scale, an estimated 4 × 1013 kilograms of carbon are annually fixed in the biosphere through the process of photosynthesis. However, it’s important to note that a considerable amount of this fixed carbon ultimately returns to the atmosphere in the form of carbon dioxide (CO2) through the respiratory activities of both producers and consumers. Additionally, decomposers make a substantial contribution to the CO2 pool by processing waste materials and the remains of deceased organic matter, whether on land or in the oceans.
Nevertheless, some of the fixed carbon becomes lost to sediments, effectively removed from circulation. Various activities further influence the release of CO2 into the atmosphere, such as the burning of wood, forest fires, combustion of organic matter, the utilization of fossil fuels, and volcanic activity.
It is of paramount importance to acknowledge that human activities have left a significant imprint on the carbon cycle. The rapid pace of deforestation, as well as the extensive burning of fossil fuels for energy production and transportation, has dramatically accelerated the rate at which carbon dioxide is released into the Earth’s atmosphere.
The Nitrogen Cycle: The Vital Element
Nitrogen Sources
Nitrogen is sourced from the atmosphere, where it exists as nitrogen gas (N2). Nitrogen-fixing bacteria convert N2 into ammonia (NH3) or nitrate (NO3-), making it available for plants.
Nitrogen Sinks
Nitrogen is stored in sinks like soil, organic matter, and oceans. Plants assimilate nitrogen from the soil, and animals obtain it by consuming plants or other animals.
The Nitrogen Cycle at Work
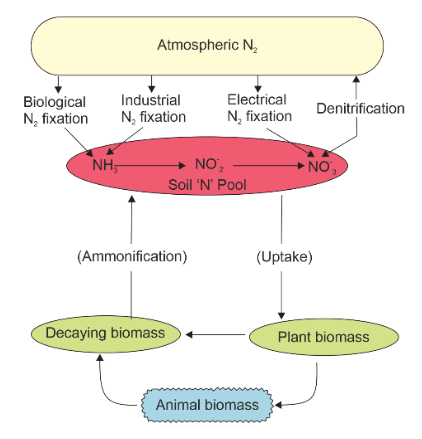
Apart from carbon, hydrogen, and oxygen, nitrogen is one of the most abundant elements found in living organisms. Nitrogen plays a crucial role in the composition of various biological molecules, including amino acids, proteins, hormones, chlorophylls, and many vitamins. In the intricate tapestry of life, plants and microbes engage in competition for the limited nitrogen available in the soil. This scarcity of nitrogen makes it a critical limiting nutrient, impacting both natural ecosystems and agricultural practices.
Nitrogen, in its molecular form, exists as two nitrogen atoms joined by a robust triple covalent bond (N≡N). The conversion of this molecular nitrogen (N2) into ammonia (NH3) is a process known as nitrogen fixation. In the natural world, phenomena like lightning and ultraviolet radiation provide the necessary energy to convert nitrogen into various nitrogen oxides, including NO (nitric oxide), NO2 (nitrogen dioxide), and N2O (nitrous oxide). Additionally, industrial processes, forest fires, automobile emissions, and power-generating facilities contribute to the presence of atmospheric nitrogen oxides.
The decomposition of organic nitrogen found in deceased plants and animals into ammonia is termed ammonification. While a portion of this ammonia may volatilize and return to the atmosphere, the majority undergoes conversion into nitrate (NO3–) through the actions of soil bacteria. The initial step involves the oxidation of ammonia to nitrite (NO2–) by specific bacteria such as Nitrosomonas and Nitrococcus. Subsequently, nitrite is further oxidized into nitrate with the assistance of another bacterium, Nitrobacter. These steps collectively constitute the process of nitrification. Notably, these nitrifying bacteria are categorized as chemoautotrophs, organisms that can produce their own energy through chemical reactions.
The nitrate produced through nitrification is absorbed by plants and transported to their leaves. Within the leaf tissues, nitrate is reduced to form ammonia, which ultimately contributes to the amine group found in amino acids. In addition to this uptake and assimilation of nitrate, nitrogen present in the soil can also be reduced back to molecular nitrogen through a process known as denitrification. Denitrification is carried out by specific bacteria, such as Pseudomonas and Thiobacillus.
In summary, nitrogen is an essential element for life, forming the building blocks of various biological molecules. Its cycling through processes like nitrogen fixation, nitrification, and denitrification is vital for sustaining ecosystems and agricultural productivity. The complex interactions between organisms and nitrogen compounds underscore the intricate web of life on our planet.
The Phosphorus Cycle: Essential for Life
Phosphorus Sources
Phosphorus primarily comes from weathered rocks and minerals. Over time, it becomes available in the soil and aquatic ecosystems.
Phosphorus Sinks
Phosphorus sinks include sediments at the bottom of bodies of water, soil, and living organisms.
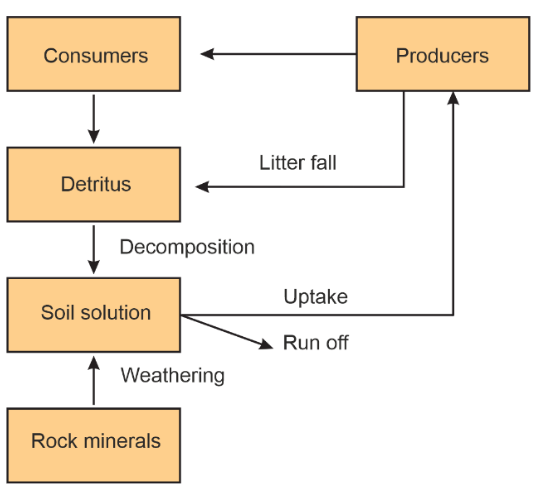
The Phosphorus Cycle in Detail
Phosphorus cycles through processes such as weathering, runoff, and the uptake of phosphate ions (PO43-) by plants. It is an essential component of DNA, RNA, and ATP.
Other Biogeochemical Cycles
Water (Hydrological) Cycle
- The water cycle is the continuous movement of water between the Earth’s surface, atmosphere, and back. It includes processes such as evaporation, condensation, precipitation, and runoff.
- Water is essential for all life forms, serving as a solvent, medium for chemical reactions, and a means of transportation for nutrients.
Sulphur Cycle
- Sulfur is essential for the synthesis of certain amino acids and vitamins. The sulfur cycle involves the movement of sulfur compounds, including hydrogen sulfide (H2S) and sulfate (SO42-), through various environmental reservoirs.
- Sulfur compounds are released into the atmosphere through volcanic eruptions and returned to the Earth’s surface through processes like deposition and bacterial sulfur reduction.
Oxygen Cycle
- The oxygen cycle is closely tied to the carbon cycle, as oxygen is a product of photosynthesis and essential for respiration in most organisms.
- Plants, algae, and cyanobacteria release oxygen during photosynthesis, while animals, including humans, consume oxygen during respiration.
Conclusion
Biogeochemical cycles are the Earth’s recycling systems, ensuring that crucial elements like carbon, nitrogen, and phosphorus are continuously available for living organisms. Understanding these cycles is vital for preserving ecosystems and addressing environmental challenges, including climate change and nutrient pollution. By appreciating the intricate dance of these elements, we gain insight into the profound interconnectedness of life on Earth.
Frequently Asked Questions on Biogeochemical Cycle
What are biogeochemical cycles?
Biogeochemical cycles, also known as nutrient cycles, are natural processes that involve the cycling of elements and compounds necessary for life through various components of the Earth's system, including living organisms, the atmosphere, and the Earth's surface.
What are the major biogeochemical cycles?
The major biogeochemical cycles include the carbon cycle, nitrogen cycle, water cycle (hydrological cycle), phosphorus cycle, and sulphur cycle. These cycles are essential for maintaining the balance of elements and sustaining life on Earth.
Why are biogeochemical cycles important?
Biogeochemical cycles are crucial for the recycling of nutrients and elements necessary for the survival of living organisms. They regulate the availability of essential elements like carbon, nitrogen, and phosphorus, which are vital for building biological molecules and sustaining ecosystems.
How do biogeochemical cycles affect the environment?
Biogeochemical cycles play a significant role in shaping ecosystems and controlling nutrient availability. Imbalances or disruptions in these cycles can lead to environmental issues such as nutrient pollution, climate change, and ecosystem degradation.
What is the carbon cycle?
The carbon cycle is the process of carbon moving between the atmosphere, living organisms, oceans, and the Earth's surface. It involves processes like photosynthesis, respiration, decomposition, and the exchange of carbon dioxide between the atmosphere and oceans.
What is the nitrogen cycle?
The nitrogen cycle is the process by which nitrogen is converted into various forms (e.g., ammonia, nitrate, nitrite) and moves between the atmosphere, soil, and living organisms. Key processes include nitrogen fixation, nitrification, denitrification, and ammonification.








