Table of Contents
Introduction to Chlorate Formula
Chlorate is an ion that contains chlorine and oxygen atoms, with the chemical formula ClO3-. It is derived from the chloric acid (HClO3) by the loss of a hydrogen ion. Chlorate ions are commonly found in various compounds and salts, such as sodium chlorate (NaClO3) and potassium chlorate (KClO3).
Chlorates are known for their strong oxidizing properties, making them useful in various applications. They are used in the production of disinfectants, bleaching agents, and explosives. Additionally, chlorates are utilized in the manufacturing of fireworks and matches, as well as in the chemical industry for the synthesis of other compounds.
Chlorate refers to any compound that contains the chlorate ion (ClO3-).
Formula and Structure of Chlorate
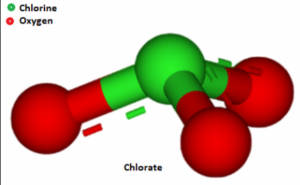
– The chlorate ion has the chemical formula ClO3-, where Cl represents chlorine and O represents oxygen.
– The ion has a trigonal pyramidal molecular geometry, with the chlorine atom at the center and three oxygen atoms surrounding it.
Physical Properties of Chlorate
– Chlorates are typically white crystalline solids.
– They are usually soluble in water, and their solubility increases with temperature.
– Chlorates can decompose upon heating, especially above their melting points.
Chemical Properties of Chlorate
– Chlorates are strong oxidizing agents and can undergo spontaneous decomposition reactions, especially when exposed to heat or when in contact with certain organic or reducing substances.
– When heated strongly, chlorates decompose to produce oxygen gas (O2) and a chloride salt.
– They can react with various reducing agents, such as sulfur, phosphorus, and organic compounds, leading to potentially explosive reactions.
Uses of Chlorate
– Chlorates have been historically used as a source of oxygen in explosives, fireworks, and matches due to their strong oxidizing properties.
– They have also been used in the production of chlorine dioxide (ClO2), a powerful bleaching agent and disinfectant.
– Chlorates find applications in the manufacture of certain chemicals, such as dyes, herbicides, and insecticides.
– In the past, chlorates were used as a weedkiller, but their use in this context has been restricted or banned in some countries due to safety concerns.
Conclusion:
In conclusion, chlorate, represented by the chemical formula ClO3-, is an ion that contains chlorine and oxygen atoms. It is derived from chloric acid and is commonly found in compounds and salts. Chlorates exhibit strong oxidizing properties and have applications in disinfection, bleaching, explosives, fireworks, and chemical synthesis. Their versatility makes chlorates significant compounds in various industries.
Solved examples on Chlorate Formulas:
Example 1: What is the formula for a compound formed when barium reacts with chlorate?
Answer: The barium cation has a charge of +2, while the chlorate anion has a charge of -1.
To balance the charges, we need two chlorate ions for every barium ion.
Therefore, the formula for the compound formed is Ba(ClO3)2.
Example 2: How many oxygen atoms are present in one molecule of calcium chlorate?
Solution: The formula for calcium chlorate is Ca(ClO3)2.
In one chlorate ion (ClO3-), there are three oxygen atoms.
Since there are two chlorate ions in the formula, we multiply the number of oxygen atoms by 2.
Therefore, there are a total of 6 oxygen atoms in one molecule of calcium chlorate.
Frequently Asked questions on Chlorate Formula
The common uses of chlorates: - Source of Oxygen: Chlorates have been used as a source of oxygen in explosives, matches, and fireworks. - Bleaching Agent and Disinfectant: Chlorates are employed in the production of chlorine dioxide (ClO2), which is a powerful bleaching agent and disinfectant. - Herbicides and Defoliants: Chlorates have historically been used as herbicides and defoliants in agriculture to control weeds and promote plant growth. However, their use in this context has been restricted or banned in some countries due to safety concerns. - Chemical Manufacturing: Chlorates find applications in the manufacture of certain chemicals, such as dyes, herbicides, and insecticides.
The another name for chlorate is Hypochlorite.
Chlorates can be synthesized through various chemical processes rather than being sourced directly from natural materials. They are typically produced by the electrolysis of a chloride salt solution, such as sodium chloride (NaCl), in the presence of an oxidizing agent. This process involves passing an electric current through the solution, resulting in the formation of chlorate ions (ClO3-) at the anode. The chlorate ions can then be isolated and further processed to obtain chlorate compounds or used directly in specific applications. It's important to note that the production of chlorates requires careful handling and control due to their oxidizing properties and potential hazards.
There are several methods that can be used to remove chlorate from water: - Activated Carbon Filtration: Activated carbon filters can effectively remove chlorate from water through adsorption. The activated carbon has a high surface area that can trap and retain chlorate molecules as water passes through the filter. - Reverse Osmosis: Reverse osmosis (RO) is a water purification process that uses a semipermeable membrane to remove impurities, including chlorate. The membrane allows water molecules to pass through while blocking larger molecules, such as chlorate ions. - Ion Exchange: Ion exchange resins can be used to remove chlorate from water. These resins have an affinity for chlorate ions and can exchange them with other ions, effectively reducing the chlorate concentration in the water. - Chemical Reduction: Chlorate can be chemically reduced to chloride using reducing agents, such as sulfur dioxide (SO2) or hydrogen peroxide (H2O2). These agents react with chlorate ions, converting them into chloride ions, which are less harmful. - Electrochemical Methods: Electrochemical processes, such as electrolysis, can be employed to remove chlorate from water. By applying an electric current, the chlorate ions can be converted into other compounds or deposited onto an electrode, effectively reducing their concentration in the water.
Chlorate can be hazardous and potentially dangerous due to its strong oxidizing properties. It can react violently with combustible materials and can pose fire and explosion risks if mishandled. Ingesting or inhaling chlorate compounds can also be harmful to human health. Therefore, it is important to handle chlorates with proper precautions and follow safety guidelines when working with or around these substances. What are the common uses of chlorate?
What is another name for chlorate?
What is the source of chlorate?
What removes chlorate from water?
Is chlorate harmful or dangerous?





