Table of Contents
A Formula is a concise and standardized mathematical or scientific expression that represents a relationship, rule, or principle. It is typically represented using symbols, variables, numbers, and mathematical operators. Formulas are used to calculate or describe various phenomena, relationships, and properties in different fields of study, such as mathematics, physics, chemistry, and engineering.
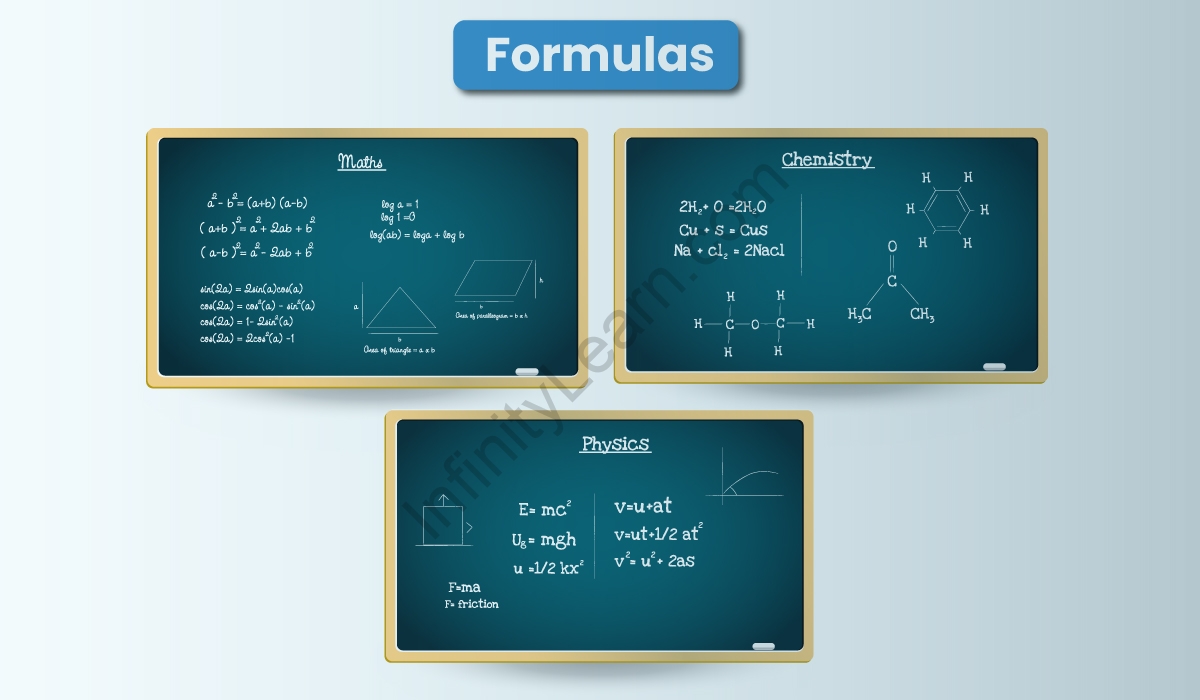
What are Formulas?
Formulas are essential in these fields because they allow scientists, engineers, and researchers to express complex concepts and calculations in a concise and standardized manner. They provide a framework for understanding and predicting the behavior of systems, solving problems, and making scientific advancements.
Subject wise Formulas
Maths Formulas
In mathematics, Formulas are used to express mathematical relationships, equations, and calculations. Math Formulas provide a way to solve problems, perform calculations, and derive new information based on given parameters.
- Algebraic Formulas
Algebraic formulas involve variables, constants, and mathematical operations such as addition, subtraction, multiplication, and division. These formulas are fundamental in algebra and serve as the building blocks for solving equations and expressing mathematical relationships.
- Geometric Formulas
Geometric formulas focus on the properties and measurements of geometric shapes and figures. They provide a means to calculate attributes such as area, perimeter, volume, angles, and side lengths. Geometric formulas are essential in geometry and trigonometry, enabling us to analyze and solve problems involving shapes and spatial relationships.
- Calculus Formulas
Calculus formulas are integral to the study of change and motion. They encompass differential and integral calculus, enabling us to analyze rates of change, find slopes, calculate areas under curves, and solve optimization problems. Calculus formulas play a vital role in fields such as physics, engineering, economics, and computer science.
- Statistical Formulas
Statistical formulas are crucial in analyzing and interpreting data. They encompass measures of central tendency, such as mean, median, and mode, as well as measures of dispersion, such as variance and standard deviation. Statistical formulas help us summarize and draw meaningful insights from data sets.
- Trigonometric Formulas
Trigonometric formulas involve ratios and relationships between angles and sides of triangles. These formulas are essential in trigonometry, enabling us to calculate angles, side lengths, and solve problems involving triangles and periodic functions.
Physics Formulas
In Physics Formulas, Formulas describe the laws of nature and mathematical relationships that govern the behavior of physical phenomena. These Formulas can be used to calculate quantities such as velocity, acceleration, force, energy, and more.
Here are the some of the important topics in Physics Formulas:
- Mechanics Formulas
Mechanics formulas form the foundation of classical physics, focusing on the motion and forces acting upon objects. They include equations for velocity, acceleration, force, energy, momentum, and Newton’s laws of motion. These formulas allow us to analyze and predict the behavior of objects in motion and understand concepts such as projectile motion and circular motion.
- Thermodynamics Formulas
Thermodynamics formulas deal with the study of heat, temperature, and energy transfer. They encompass equations related to the laws of thermodynamics, heat transfer mechanisms, and calculations of work, internal energy, entropy, and efficiency. Thermodynamics formulas are essential in understanding concepts such as heat engines, refrigeration, and the behavior of gases.
- Electromagnetism Formulas
Electromagnetism formulas describe the interplay between electricity and magnetism. They include equations for electric fields, magnetic fields, electromagnetic waves, electric circuits, and electromagnetic induction. These formulas are crucial in understanding phenomena like electric potential, electromagnetic radiation, electromagnetic motors, and generators.
- Optics Formulas
Optics formulas focus on the behavior of light and its interaction with matter. They encompass equations for reflection, refraction, lenses, mirrors, interference, diffraction, and optical instruments. Optics formulas allow us to comprehend the characteristics of light, image formation, and phenomena like color perception and optical illusions.
- Quantum Mechanics Formulas
Quantum mechanics formulas delve into the realm of the microscopic, describing the behavior of particles at the quantum level. They include equations for wave-particle duality, energy quantization, probability distributions, and wavefunctions. Quantum mechanics formulas are vital in understanding the behavior of atoms, subatomic particles, and phenomena like quantum entanglement and superposition.
Chemistry Formulas
In chemistry, Formulas are used to represent chemical compounds and reactions. Chemistry Formulas represent the composition of substances, while equations show the interaction and transformation of substances during chemical reactions.
- Empirical Formulas
Empirical formulas represent the simplest ratio of atoms in a compound. They provide information about the relative number of atoms of each element present in a molecule. Empirical formulas are derived from experimental data, such as mass or percentage composition, and are essential for understanding the stoichiometry of chemical reactions.
- Molecular Formulas
Molecular formulas specify the actual number of atoms of each element in a molecule. They provide a more detailed representation of a compound’s composition compared to empirical formulas. Molecular formulas allow us to identify and differentiate between different compounds with the same empirical formula.
- Structural Formulas
Structural formulas depict the arrangement of atoms within a molecule and the bonds between them. They provide a visual representation of the connectivity and spatial orientation of atoms in a compound. Structural formulas are crucial for understanding the three-dimensional structure and properties of molecules.
- Balanced Chemical Equations
Balanced chemical equations represent the conservation of mass and atoms in chemical reactions. They provide a concise summary of the reactants, products, and stoichiometry of a reaction. Balanced chemical equations allow us to calculate quantities, predict reaction outcomes, and understand the underlying principles of chemical transformations.
- Lewis Dot Structures
Lewis dot structures depict the valence electrons of atoms in a molecule using dots or lines. They provide a simplified representation of electron distribution and help us understand the bonding and geometry of molecules. Lewis dot structures are essential for predicting molecular properties, such as polarity and reactivity.
Importance of Formula:
Formulas play a significant role in mathematics as they provide concise representations of mathematical relationships, equations, and calculations.
Here are a few key points highlighting the significance of formulas in mathematics:
- Problem-solving: Formulas serve as powerful tools for problem-solving. They allow mathematicians to translate real-world problems into mathematical equations, making it easier to analyze and find solutions.
- Efficiency: Formulas condense complex mathematical concepts into concise expressions, enabling efficient computation and calculation. They provide a shortcut for performing repetitive calculations, saving time and effort.
- Communication: Formulas facilitate communication in mathematics by providing a common language for expressing mathematical ideas and concepts. They allow mathematicians to communicate precise mathematical relationships and results to others in a compact and understandable manner.
- Generalization: Formulas often represent general principles or patterns in mathematics. They enable mathematicians to generalize concepts and apply them to various situations, expanding the scope of mathematical knowledge.
- Derivation: Formulas can be derived using mathematical reasoning and proof techniques. They help in deducing new relationships, properties, and theorems based on existing mathematical knowledge.
- Bridging theory and application: Formulas bridge the gap between theoretical concepts and their practical applications. They provide a framework for applying mathematical principles to solve real-world problems in fields such as physics, engineering, economics, and more.
FAQs on List of Formulas
What is a simple Formulas?
A simple Formulas is a basic mathematical expression that involves a limited number of variables, operations, and functions. It typically represents a straightforward calculation or relationship. Simple Formulas are often used to perform elementary calculations or solve basic mathematical problems.
What are the 4 types of math?
The four main branches of mathematics are algebra, geometry, calculus, and statistics. Algebra deals with symbols and the rules for manipulating them, while geometry focuses on the properties and relationships of shapes. Calculus is concerned with rates of change and accumulation, and statistics involves the collection, analysis, interpretation, and presentation of data.
What is Formulas in chemistry?
Formulas in chemistry refers to the plural form of Formulas. It is used when referring to multiple chemical Formulas. A chemical Formulas is a concise representation of the elements and their ratios in a compound. For example, the chemical Formulas for water is H₂O, which indicates that it consists of two hydrogen atoms (H) and one oxygen atom (O).
Which is the correct Formulas or Formulas?
Both Formulas and Formulas are accepted plural forms of the word Formulas. However, Formulas is more commonly used in American English, while Formulas is often preferred in British English. Both forms are correct and can be used interchangeably, depending on the regional or stylistic conventions followed.
What are the types of Formulas?
There are different types of Formulas based on the field of study. In mathematics, common types include algebraic Formulas, geometric Formulas, and trigonometric Formulas. In chemistry, Formulas can be molecular Formulas, empirical Formulas, or structural Formulas. In physics, Formulas encompass laws and principles such as Newton's laws, Ohm's law, and the laws of thermodynamics. The types of Formulas vary depending on the subject, and each type serves a specific purpose in representing relationships, calculations, or principles within their respective fields
What is the significance of stoichiometric formulas in chemistry?
Stoichiometric formulas provide crucial information about the quantitative relationship between reactants and products in a chemical reaction. They help determine the exact amount of substances needed for a reaction and predict the yield of products. By using stoichiometric formulas, chemists can design efficient synthesis routes, optimize reaction conditions, and ensure the proper utilization of resources
How are empirical formulas different from molecular formulas?
Empirical formulas represent the simplest whole-number ratio of atoms in a compound, while molecular formulas indicate the actual number of atoms of each element present in a molecule. Empirical formulas provide a general understanding of a compound's composition, while molecular formulas offer a more precise depiction. For example, the empirical formula of hydrogen peroxide is HO, while its molecular formula is H₂O₂, indicating that each molecule contains two hydrogen atoms and two oxygen atoms.








