Ozone is an inorganic molecule composed of three oxygen atoms bonded together. It is a pale blue gas that has a distinct odor and is present in the Earth’s atmosphere. The formula for ozone is O3.
Ozone is formed in the Earth’s atmosphere through the photolysis of oxygen molecules (O2) by ultraviolet (UV) radiation.
It is important to note that the presence of ozone in the atmosphere is essential for maintaining the balance of the Earth’s ecosystems, but its concentration and distribution need to be carefully monitored to ensure environmental and human health.
Structural Formula of Ozone
The molecular formula O3 indicates that ozone consists of three oxygen atoms. Each oxygen atom in the ozone molecule is bonded to the other two oxygen atoms through double bonds, resulting in a cyclic structure. This arrangement gives ozone its unique properties and reactivity.
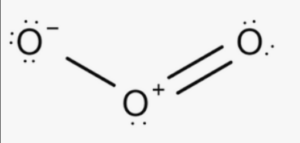
In this representation, the lines between the oxygen atoms represent the double bonds, indicating that each oxygen atom shares two pairs of electrons with the neighboring oxygen atoms.The presence of double bonds in the ozone molecule makes it relatively unstable and reactive.
Uses of Ozone
- Water treatment: Ozone is used as a disinfectant in water treatment processes. It can kill bacteria, viruses, and other microorganisms, making it effective in water purification and wastewater treatment.
- Air purification: Ozone generators are used to remove odors, bacteria, and other contaminants from the air. Ozone reacts with pollutants and breaks them down, improving air quality.
- Industrial applications: Ozone is used in various industrial processes, such as chemical synthesis, bleaching of textiles and paper, and removal of pollutants from industrial emissions.
- Medical applications: Ozone therapy, which involves the controlled medical use of ozone, has been used for various purposes, including wound healing, treatment of certain infections, and stimulation of the immune system.
- Food preservation: Ozone can be used to extend the shelf life of fruits, vegetables, and other perishable foods. It can inhibit the growth of bacteria, molds, and yeast, helping to prevent spoilage.
- Laboratory applications: Ozone is used in laboratory settings for sterilization, cleaning of equipment, and oxidation reactions.
Physical Properties of Ozone Formula
- State: Ozone is a gas at room temperature and atmospheric pressure. It exists as a pale blue gas with a characteristic pungent odor.
- Density: The density of ozone gas is higher than that of oxygen gas (O2). Ozone has a density of about 2.14 kg/m³, which is higher than the density of air.
- Melting Point: Ozone has a low melting point of -192.2°C (-314.0°F). At temperatures below its melting point, ozone solidifies into dark blue crystals.
- Boiling Point: Ozone does not have a distinct boiling point because it decomposes before reaching its boiling point. However, it starts to decompose into oxygen gas at temperatures above -112°C (-170°F).
- Solubility: Ozone is moderately soluble in water. It dissolves in water, forming a weakly acidic solution called ozonated water.
- Stability: Ozone is a relatively unstable molecule and readily decomposes back into oxygen. It is highly reactive and can react with various substances, including organic compounds and pollutants.
- Reactivity: Ozone is a powerful oxidizing agent. It reacts with many substances, including metals, organic compounds, and atmospheric pollutants. This reactivity makes it effective for various applications, such as disinfection, water treatment, and air purification.
- Odor: Ozone has a distinctive odor often described as “fresh” or “sharp.” It is noticeable even at low concentrations and can be detected near electrical equipment or during thunderstorms, where ozone is formed by lightning.
- Toxicity: Ozone is toxic and can be harmful to living organisms. Inhalation of high concentrations of ozone can irritate the respiratory system, cause breathing difficulties, and damage lung tissue. It is important to handle ozone with caution and ensure proper ventilation.
Chemical Properties of Ozone Formula
- Oxidizing Agent: Ozone is a potent oxidizing agent, meaning it has the ability to accept electrons from other substances during chemical reactions. It can oxidize a wide range of compounds, including organic compounds, metals, and inorganic substances. Its strong oxidative properties make it effective in disinfection and sterilization applications.
- Reactive with Double Bonds: Ozone readily reacts with compounds containing double bonds, such as alkenes. It can undergo a reaction called ozonolysis, where the ozone molecule breaks the double bond and forms ozonides (ozonolysis products). This reaction is used in organic chemistry to identify the presence and location of double bonds in compounds.
- Decomposition: Ozone is unstable and can decompose spontaneously into oxygen (O2). This process can occur through different pathways, including thermal decomposition and photolysis. The decomposition of ozone is accelerated by heat, light, and catalysts, such as metal surfaces or certain gases.
- Acidic Nature: Ozone is a weak acid, and when dissolved in water, it forms a weak acid known as ozonated water or aqueous ozone. This leads to the formation of hydrogen ions (H+) and the generation of a mildly acidic environment.
- Reaction with Nitrogen Oxides: Ozone can react with nitrogen oxides (NOx), typically produced by combustion processes, to form nitrogen dioxide (NO2). This reaction is a part of atmospheric chemistry and contributes to the formation of smog and photochemical air pollution.
- Sensitivity to UV Radiation: Ozone is sensitive to ultraviolet (UV) radiation. When UV radiation interacts with ozone in the stratosphere, it undergoes photodissociation, resulting in the formation of oxygen molecules (O2) and oxygen atoms (O). This process plays a crucial role in the formation and depletion of the ozone layer.
Conclusion
In conclusion, the formula for ozone is O3. Ozone is a highly reactive molecule consisting of three oxygen atoms. It has various applications and uses in water treatment, air purification, industrial processes, medical treatments, food preservation, and laboratory settings. Ozone’s ability to disinfect, remove odors, and break down pollutants makes it valuable in these applications. However, it is important to handle ozone with caution due to its potential hazards at high concentrations. Overall, the formula for ozone represents a powerful and versatile molecule with significant practical implications.
Solved Examples on Ozone Formula
Example 1: Calculation of Ozone Concentration
A water treatment plant is using ozone for disinfection purposes. The plant wants to determine the concentration of ozone in the water after the ozone gas is dissolved. Given the following information:
- Volume of water: 500 liters
- Ozone gas flow rate: 10 grams per minute
- Ozone gas density: 2.144 grams per liter
Solution:
Step 1: Calculate the total mass of ozone gas used.
Mass = flow rate × time
Mass = 10 g/min × 1 min
Mass = 10 grams
Step 2: Calculate the volume of ozone gas used.
Volume = mass / density
Volume = 10 g / 2.144 g/L
Volume ≈ 4.66 liters
Step 3: Calculate the concentration of ozone in the water.
Concentration = Volume of ozone gas / Volume of water
Concentration = 4.66 liters / 500 liters
Concentration ≈ 0.00932 liters of ozone per liter of water
Therefore, the concentration of ozone in the water is approximately 0.00932 liters of ozone per liter of water.
Example 2: Calculating the Amount of Ozone Required for Air Treatment
A facility requires ozone for air treatment to remove odors. The target concentration of ozone in the air is 0.04 parts per million (ppm), and the volume of the room that needs treatment is 1000 cubic meters. Given the following information:
- Ozone has a molar mass of approximately 48 grams per mole.
- The ideal gas law can be used to calculate the number of moles.
Solution:
Step 1: Convert the target concentration of ozone from ppm to moles per cubic meter.
0.04 ppm = 0.04 parts per million = 0.04/1,000,000 = 4 × 10-8 moles per mole of air
Step 2: Calculate the number of moles of ozone needed.
Number of moles = Concentration × Volume
Number of moles = 4 × 10-8 moles per mole of air × 1000 cubic meters
Number of moles = 4 × 10-5 moles of ozone
Step 3: Convert the number of moles to grams.
Mass = Number of moles × Molar mass
Mass = 4 × 10-5 moles × 48 grams per mole
Mass = 1.92 × 10-3 grams of ozone
Therefore, approximately 1.92 milligrams of ozone is required for air treatment in the given facility.
Frequently Asked Questions on Ozone Formula
1: How is ozone formula formed?
Answer: Ozone (O3) is formed through a series of chemical reactions involving oxygen molecules (O2) and energy, typically in the presence of ultraviolet (UV) radiation. The formation of ozone can be summarized in the following steps:
- Absorption of UV Radiation: When high-energy UV radiation from the sun reaches the Earth’s stratosphere, some of it is absorbed by oxygen molecules (O2).
- Formation of Oxygen Atoms: The absorbed UV radiation causes the oxygen molecules to break apart, resulting in the dissociation of oxygen molecules into individual oxygen atoms (O).
O2 + UV radiation → 2O
- Reaction of Oxygen Atoms with Oxygen Molecules: The highly reactive oxygen atoms (O) can then react with other oxygen molecules (O2) to form ozone (O3). This reaction occurs through a combination of collision and energy transfer processes.
O + O2 → O3
Overall, the formation of ozone involves the conversion of oxygen molecules (O2) into ozone molecules (O3) through the presence of UV radiation and the involvement of oxygen atoms (O). The process is cyclic, with ozone continuously being formed and decomposed in the stratosphere.
2: Who discovered the formula of ozone?
Answer: Ozone is a naturally occurring substance which was first made in the laboratory in 1839 by German scientist Christian Friedrich Schönbein.
3: What chemical breaks down ozone?
Answer: Chlorofluorocarbons (CFCs) and certain other halogenated compounds are primarily responsible for the breakdown of ozone in the Earth’s atmosphere. These compounds contain chlorine (Cl) and bromine (Br) atoms, which can catalytically destroy ozone molecules. The chemical reactions involved in ozone depletion are known as ozone-depleting reactions.
4: Who named ozone hole?
Answer: The term “ozone hole” was coined by scientists at the British Antarctic Survey (BAS) in the mid-1980s. In particular, the British scientists Joe Farman, Brian Gardiner, and Jonathan Shanklin were studying the depletion of ozone in the stratosphere over Antarctica.
They noticed a significant and alarming decrease in ozone concentrations in the region during the springtime of the Southern Hemisphere. To describe this phenomenon, they used the term “ozone hole” to convey the severe depletion of ozone in that specific area.
5: Which layer is harmful ozone located?
Answer: Harmful ozone, commonly known as ground-level ozone, is not located in a specific layer of the atmosphere but rather exists in the troposphere, which is the lowest layer of the Earth’s atmosphere extending approximately 7 to 20 kilometers (4 to 12 miles) above the Earth’s surface. The troposphere is where weather occurs and where we live and breathe.
Ground-level ozone is formed through chemical reactions involving pollutants emitted by human activities, such as vehicle emissions, industrial processes, and the interaction of sunlight with certain pollutants. These reactions produce ozone at ground level, leading to the formation of smog, which can have harmful effects on human health and the environment.
6: Is ozone harmful to humans?
Answer: Ozone can be harmful to humans at high concentrations. It can irritate the respiratory system, cause breathing difficulties, and worsen existing respiratory conditions. However, at lower concentrations typically found in the atmosphere, ozone plays a crucial role in protecting the Earth from harmful ultraviolet (UV) radiation.
7: What is in Ozone?
Answer: Ozone (O3) is a molecule composed of three oxygen atoms bonded together. Each oxygen atom in ozone is connected to the other two by a double bond, resulting in a bent molecular structure. This arrangement gives ozone its unique chemical and physical properties.
Ozone is a pale blue gas that is formed in the Earth’s atmosphere through the interaction of sunlight with oxygen molecules (O2). It is also produced artificially through various methods, such as electric discharge or ultraviolet light.
The molecular formula for ozone, O3, represents its composition, where one molecule consists of three oxygen atoms. The oxygen atoms in ozone are highly reactive, and the molecule has a strong oxidizing potential. This reactivity makes ozone effective in various applications, such as air purification, water treatment, and disinfection.
In the Earth’s atmosphere, ozone plays a crucial role in the ozone layer, which is a protective layer that absorbs and filters out much of the Sun’s harmful ultraviolet (UV) radiation. However, at ground level, high concentrations of ozone can be harmful to human health and the environment.
It is important to note that while ozone has beneficial uses, it should be handled and used with caution, following appropriate safety guidelines and regulations.
8: Why is ozone a pollutant?
Answer: Ozone is considered a pollutant when it is present in high concentrations in the lower atmosphere, near the Earth’s surface. It is formed through chemical reactions involving pollutants emitted by human activities, such as vehicle emissions and industrial processes. High levels of ground-level ozone can be harmful to human health, cause damage to vegetation, contribute to poor air quality, and have warming effects on the climate.




