Table of Contents
Glycerol, also known as glycerin or glycerine, is a colourless and odourless liquid with a sweet taste. It has the chemical formula C3H8O3 and belongs to the alcohol functional group. Glycerol is a trihydroxy sugar alcohol, meaning it contains three hydroxyl (-OH) groups attached to a three-carbon backbone.
Uses of Glycerol
- Personal Care and Cosmetics: Glycerol is a common ingredient in skincare products, such as moisturizers, lotions, and creams. It helps to hydrate and soften the skin, acting as a humectant that attracts and retains moisture. Glycerol is also used in hair care products, soaps, and bath products.
- Food and Beverages: Glycerol is used as a sweetener and humectant in the food and beverage industry. It provides sweetness without contributing to tooth decay and helps to retain moisture in products like baked goods, confectionery, and beverages. Glycerol is also used as a thickening agent, stabilizer, and preservative in certain food products.
- Pharmaceuticals: Glycerol has pharmaceutical applications and is used as a solvent, co-solvent, and lubricant in the production of oral and topical medications. It can also serve as a preservative in certain pharmaceutical formulations.
- Industrial Applications: Glycerol is used in various industrial processes, including the production of explosives, polyurethane foams, and antifreeze solutions. It is also used as a solvent and additive in the manufacturing of paints, inks, and coatings.
- E-Cigarettes and Vaping: Glycerol is commonly used as a component in e-liquids for electronic cigarettes and vaping devices. It helps to create vapor and provides a smoother inhaling experience.
Structural Formula of Glycerol
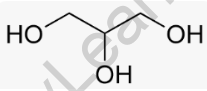
In this structure, each “O” represents an oxygen atom, “H” represents a hydrogen atom, and the dashes (-) represent the covalent bonds between the atoms. Glycerol is a trihydroxy sugar alcohol, meaning it has three hydroxyl (-OH) groups attached to a three-carbon backbone.
Physical Properties of Glycerol
- Physical State: Glycerol is a clear, colorless, viscous liquid at room temperature.
- Odor and Taste: Glycerol has a sweet taste and a mild, characteristic odor.
- Density: The density of glycerol is approximately 1.26 g/mL.
- Boiling Point: Glycerol has a boiling point of approximately 290°C (554°F).
- Melting Point: Glycerol has a melting point of approximately 17.8°C (64°F).
- Solubility: Glycerol is highly soluble in water, meaning it readily dissolves in water to form a homogeneous solution.
- Viscosity: Glycerol has a high viscosity, which means it flows slowly and has a thick consistency.
- Refractive Index: The refractive index of glycerol is approximately 1.47.
- Hygroscopic Nature: Glycerol is hygroscopic, meaning it has the ability to absorb and hold moisture from the surrounding environment.
Chemical Properties of Glycerol
- Hydroxyl Groups: Glycerol contains three hydroxyl (-OH) groups. These hydroxyl groups make glycerol a versatile compound that can undergo various chemical reactions.
- Alcohol Reactivity: Glycerol is classified as a triol, which means it has three alcohol groups. These alcohol groups can undergo reactions such as esterification, oxidation, and reduction.
- Ester Formation: Glycerol can react with organic and inorganic acids to form esters. This property is utilized in the production of various esters for applications in the food, cosmetic, and pharmaceutical industries.
- Oxidation: Glycerol can undergo oxidation reactions to form compounds such as glyceric acid and dihydroxyacetone. These oxidation reactions are often catalyzed by enzymes or chemical oxidizing agents.
- Dehydration: Under certain conditions, glycerol can undergo dehydration reactions to form acrolein, a volatile and highly reactive compound. This property is important in the production of acrylic acid and other chemicals.
- Reactivity with Nitric Acid: Glycerol can react with concentrated nitric acid to form nitroglycerin, an explosive compound. This reaction is highly exothermic and requires careful handling.
- Stability: Glycerol is a stable compound under normal conditions. It is non-volatile and does not readily undergo spontaneous decomposition or reactions.
| Also Check | |
| Ethylene glycol formula | Bicarbonate formula |
| Ammonium nitrate formula | Ammonium hydroxide formula |
Conclusion
In conclusion, glycerol, also known as glycerin or glycerine, is a versatile compound with various applications. It is widely used in personal care and cosmetics for its moisturizing properties, in the food and beverage industry as a sweetener and humectant, in pharmaceuticals as a solvent and lubricant, and in industrial processes for the production of explosives, coatings, and polyurethane foams. Glycerol’s trihydroxy sugar alcohol structure, along with its chemical reactivity, allows it to participate in ester formation, oxidation, and dehydration reactions. It is a stable compound with a high viscosity and hygroscopic nature. Glycerol plays a significant role in numerous industries due to its beneficial properties and diverse range of applications.
Solved Examples on Glycerol Formula
Example 1: Calculate the molar mass of glycerol (C3H8O3) and determine the number of moles in 250 grams of glycerol.
Solution: Determine the molar mass of glycerol:
- C: Atomic mass of carbon = 12.01 g/mol
- H: Atomic mass of hydrogen = 1.01 g/mol
- O: Atomic mass of oxygen = 16.00 g/mol
Molar mass of glycerol = (3 * 12.01) + (8 * 1.01) + (3 * 16.00) = 92.09 g/mol
Calculate the number of moles:
Given mass of glycerol = 250 grams
Molar mass of glycerol = 92.09 g/mol
Number of moles = Mass / Molar mass = 250 g / 92.09 g/mol = 2.72 mol
Therefore, there are approximately 2.72 moles of glycerol in 250 grams of glycerol.
Example 2: A 500 mL solution contains 30 grams of glycerol (C3H8O3). Calculate the molarity of glycerol in the solution.
Solution: Calculate the number of moles of glycerol:
Given mass of glycerol = 30 grams Molar mass of glycerol = (3 * 12.01) + (8 * 1.01) + (3 * 16.00) = 92.09 g/mol
Number of moles of glycerol = Mass / Molar mass = 30 g / 92.09 g/mol ≈ 0.3257 mol
Convert the volume of the solution to liters: Given volume of the solution = 500 mL
= 500/1000 L = 0.5 L
Calculate the molarity of glycerol:
Molarity (M) = Number of moles / Volume in liters
= 0.3257 mol / 0.5 L = 0.6514 M
Therefore, the molarity of glycerol in the 500 mL solution is approximately 0.6514 M.
Frequently Asked Questions on Glycerol Formula
What is unique about glycerol?
Glycerol was accidentally discovered by a Swedish scientist named K. W. Scheele. Scheele analyzed the substance and found it to be distinct from the other sugars known at the time. Glycerol did not crystallize, ferment, and showed greater heat resistance than most other sugars.
Why is glycerol attracted to water?
Glycerol is attracted to water due to its hydrophilic (water-loving) nature. Glycerol is a polar molecule, meaning it has a partial positive charge on one end and a partial negative charge on the other end. This polarity arises from the presence of hydroxyl (-OH) groups in its molecular structure. Water molecules are also polar, with oxygen being partially negative and hydrogen being partially positive. The partial positive charge of water molecules is attracted to the partial negative charge of glycerol, and vice versa. This electrostatic attraction between the polar molecules allows glycerol to mix well with water and dissolve easily.
What is another name for glycerol?
Glycerol is also commonly known as glycerin or glycerine. These terms are used interchangeably to refer to the same compound, which is a colourless, odourless, and viscous liquid.
What is the source of glycerol?
Glycerol can be derived from various sources, including plant and animal fats. In the production of glycerol, fats and oils are hydrolyzed (broken down) into their constituent fatty acids and glycerol molecules. The glycerol obtained from this process can then be further purified and used for various applications. Additionally, glycerol can also be synthesized through chemical processes, such as the hydrolysis of propylene.








