Table of Contents
Oxalic acid is a dicarboxylic acid with the chemical formula (COOH)2 or C2H2O4. It is an organic compound that occurs naturally in various plants and can be synthesized industrially. Oxalic acid is notable for its strong acidity and diverse applications. Let’s explore its formula, structure, and physical and chemical properties:
Formula of Oxalic Acid
The chemical formula of oxalic acid is (COOH)2 or C2H2O4. It consists of two carboxyl (COOH) groups, indicating the presence of two acidic functional groups in the molecule.
Structure of Oxalic Acid
The structure of oxalic acid can be represented as:
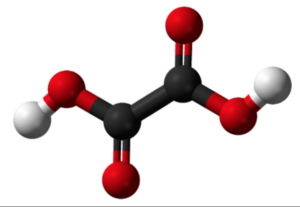
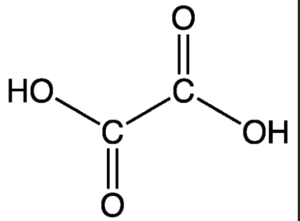
In the structure, the two carboxyl groups are attached to a central carbon atom. Each carboxyl group consists of a carbon atom bonded to two oxygen atoms (one through a double bond and another through a single bond) and a hydrogen atom.
Physical Properties of Oxalic Acid
- State: Oxalic acid is a white crystalline solid at room temperature.
- Odor: It is odorless.
- Melting Point: The melting point of oxalic acid is around 189-191 degrees Celsius (372-376 degrees Fahrenheit).
- Solubility: Oxalic acid is highly soluble in water. It forms a clear, colorless solution.
Chemical Properties of Oxalic Acid
- Acidity: Oxalic acid is a strong acid, dissociating into two hydrogen ions (H+) per molecule. It readily donates protons in chemical reactions.
- Reducing Agent: Oxalic acid can act as a reducing agent, undergoing oxidation itself. It can reduce various metal ions, such as ferric ions (Fe3+) to ferrous ions (Fe2+).
- Chelating Agent: Due to its ability to form stable complexes with metal ions, oxalic acid acts as a chelating agent. It forms water-soluble complexes with many metal ions, aiding in their extraction and purification.
- Oxidation Reactions: Oxalic acid can be oxidized by strong oxidizing agents, such as potassium permanganate (KMnO4), resulting in the formation of carbon dioxide (CO2) and water (H2O).
Applications of Oxalic Acid
- Cleaning and Metal Polishing: Oxalic acid is commonly used as a cleaning agent for rust and stains. It can remove rust from metals and is effective in polishing and brightening metal surfaces.
- Bleaching Agent: Oxalic acid is used in certain bleaching applications, such as in textile and wood industries.
- Pharmaceutical and Chemical Industry: It finds applications in the synthesis of various pharmaceuticals, dyes, and chemicals.
- Coordination Chemistry: Oxalic acid is widely used in coordination chemistry due to its ability to form stable complexes with metal ions.
Oxalic acid, with its distinct formula, structure, and properties, plays a significant role in various industries and chemical processes. Understanding these aspects is crucial for its safe handling and its applications in different fields.
Solved Examples on Oxalic acid
Example 1: Calculate the molar mass of oxalic acid (C₂H₂O₄).
Solution: To calculate the molar mass of oxalic acid, we need to determine the atomic masses of carbon (C), hydrogen (H), and oxygen (O) and add them up.
Atomic mass of carbon (C) = 12.01 g/mol
Atomic mass of hydrogen (H) = 1.01 g/mol
Atomic mass of oxygen (O) = 16.00 g/mol
Since oxalic acid (C2H2O4) consists of two carbon atoms, two hydrogen atoms, and four oxygen atoms, we multiply the atomic masses of carbon, hydrogen, and oxygen by their respective coefficients and add them up:
Molar mass of oxalic acid (C2H2O4) = (2 × atomic mass of carbon) + (2 × atomic mass of hydrogen) + (4 × atomic mass of oxygen)
= (2 × 12.01 g/mol) + (2 × 1.01 g/mol) + (4 × 16.00 g/mol)
= 24.02 g/mol + 2.02 g/mol + 64.00 g/mol
= 90.04 g/mol
Therefore, the molar mass of oxalic acid (C2H2O4) is 90.04 g/mol.
Example 2: Determine the number of moles in 50 grams of oxalic acid (C2H2O4).
Solution: To find the number of moles in a given mass of oxalic acid, we need to use the molar mass of oxalic acid.
Molar mass of oxalic acid (C2H2O4) = 90.04 g/mol (as calculated in Example 1)
Number of moles = Mass of substance / Molar mass
= 50 g / 90.04 g/mol
≈ 0.55 moles
Therefore, there are approximately 0.55 moles of oxalic acid (C2H2O4) in 50 grams.
Frequently Asked Questions on Oxalic acid
What is the chemical formula of oxalic acid?
The chemical formula of oxalic acid is C2H2O4.
What are the elements present in the formula of oxalic acid?
The formula of oxalic acid consists of two carbon C atoms, two hydrogen H atoms, and four oxygen O atoms.
Is oxalic acid an organic or inorganic compound?
Oxalic acid is an organic compound, as it contains carbon atoms bonded to hydrogen atoms.
What is the structure of oxalic acid?
The structure of oxalic acid consists of two carboxyl COOH groups attached to a central carbon atom. Each carboxyl group contains a carbon atom bonded to two oxygen atoms and a hydrogen atom.
What is the molar mass of oxalic acid?
The molar mass of oxalic acid is approximately 90.04 grams per mole g/mol. This can be calculated by summing up the atomic masses of its constituent elements.








