Table of Contents
Potassium Nitrate Formula
Introduction
Potassium nitrate, also known as saltpeter or nitre, is a chemical compound with the formula KNO3. It is a crystalline salt that is composed of potassium ions (K+) and nitrate ions (NO3-).
Potassium nitrate is commonly used in various industries and applications, including as a fertilizer, oxidizer in fireworks, food preservative, and component in gunpowder. It has been historically used in the production of gunpowder and as a key ingredient in the manufacture of explosives. Potassium nitrate is a versatile compound with several important uses in agriculture, industry, and chemistry.
Uses of Potassium Nitrate
- Fertilizer: Potassium nitrate is a significant source of potassium and nitrogen, essential nutrients for plant growth. It is used as a fertilizer in agriculture to promote healthy plant development, improve crop yields, and enhance overall soil fertility.
- Food Preservation: Potassium nitrate is sometimes used as a food preservative due to its antimicrobial properties. It can help inhibit the growth of bacteria and other microorganisms, extending the shelf life of certain food products, particularly cured meats.
- Pyrotechnics: Potassium nitrate is a key ingredient in the production of fireworks, black powder, and other pyrotechnic devices. It serves as an oxidizing agent, supplying oxygen for the combustion of other compounds and contributing to the vibrant colors and explosive effects of fireworks.
- Gunpowder and Explosives: Historically, potassium nitrate has been a vital component in the production of gunpowder and explosives. Its oxidizing properties, combined with sulfur and charcoal, create a highly combustible mixture.
- Oxidizer: Potassium nitrate is used as an oxidizing agent in various industrial processes, such as in the manufacture of matches, fertilizers, and certain chemicals. It provides oxygen to support combustion and chemical reactions.
- Oral Care: Potassium nitrate is sometimes included in toothpaste and mouthwash formulations. It may help alleviate tooth sensitivity by desensitizing the nerves in the teeth. It is commonly used in oral care products for its potential therapeutic benefits.
- Heat Transfer Fluid: Potassium nitrate can be used as a heat transfer medium in some solar power systems and thermal storage applications. Its high specific heat capacity and thermal stability make it suitable for storing and releasing thermal energy.
Structural Formula of Potassium Nitrate
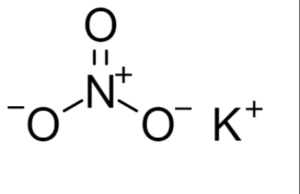
In this structure, the potassium ion (K+) is bonded to the nitrate ion (NO3-). The nitrogen atom (N) forms three covalent bonds with three oxygen atoms (O), and one of the oxygen atoms carries a negative charge.
Also Check: Zinc Nitrate Formula
Physical Properties of Potassium Nitrate Formula
- Appearance: Potassium nitrate is a crystalline solid that typically appears as colorless or white crystals.
- Melting and Boiling Point: The melting point of potassium nitrate is approximately 334 degrees Celsius (633 degrees Fahrenheit), and it does not have a distinct boiling point. Instead, it decomposes when heated to release oxygen and nitrogen oxides.
- Solubility: Potassium nitrate is highly soluble in water. It forms a clear and colorless solution when dissolved.
- Density: The density of potassium nitrate is about 2.11 grams per cubic centimeter (g/cm3).
- Odour and Taste: Potassium nitrate is odorless and has a slightly salty taste.
- Hygroscopicity: Potassium nitrate has hygroscopic properties, meaning it can absorb moisture from the air.
- Crystal Structure: Potassium nitrate crystallizes in the orthorhombic crystal system, forming elongated prismatic crystals.
Chemical Properties of Potassium Nitrate Formula
- Oxidizing Agent: Potassium nitrate is a powerful oxidizing agent, meaning it can donate oxygen to support combustion reactions. It is commonly used in fireworks, explosives, and as an oxidizer in rocket propellants.
- Decomposition: When heated, potassium nitrate decomposes into potassium nitrite (KNO2) and oxygen gas (O2). This decomposition reaction is highly exothermic and releases a significant amount of heat. 2KNO3(s) → 2KNO2(s) + O2(g)
- Solubility: Potassium nitrate is highly soluble in water, which allows it to easily dissolve and form a solution. This property is utilized in various applications such as fertilizers and in the preparation of salt solutions.
- Reaction with Acids: Potassium nitrate reacts with acids to form nitric acid and corresponding potassium salt. For example, when reacted with sulfuric acid (H2SO4), it forms potassium sulfate (K2SO4) and nitric acid (HNO3). KNO3(s) + H2SO4(l) → K2SO4(s) + HNO3(l)
- Reaction with Metals: Potassium nitrate can react with certain metals, such as magnesium or aluminum, in the presence of heat to undergo a combustion reaction. This reaction produces metal oxides and releases nitrogen gas. 2KNO3(s) + 3Mg(s) → MgO(s) + N2(g) + 2K2O(s)
- pH: Potassium nitrate is a neutral compound and does not significantly affect the pH of a solution when dissolved in water.
Solved Examples on Potassium Nitrate Formula
Example 1: Combustion Reaction
What mass of oxygen gas is required for the complete combustion of 50 grams of potassium nitrate?
Solution:
- Determine the molar mass of potassium nitrate (KNO3):
Molar mass of K = 39.10 g/mol
Molar mass of N = 14.01 g/mol
Molar mass of O = 16.00 g/mol
Molar mass of KNO3 = (39.10 + 14.01 + (3 * 16.00)) g/mol
= 101.10 g/mol
- Convert the given mass of potassium nitrate to moles:
Moles of KNO3 = Mass / Molar mass
Moles of KNO3 = 50 g / 101.10 g/mol ≈ 0.494 moles
- Determine the stoichiometric ratio between potassium nitrate and oxygen gas from the balanced equation:
2KNO3 → 2KNO2 + O2
From the equation, 2 moles of potassium nitrate produce 1 mole of oxygen gas.
- Calculate the moles of oxygen gas required:
Moles of O2 = 0.494 moles / 2 ≈ 0.247 moles
- Convert the moles of oxygen gas to mass:
Mass of O2 = Moles of O2 * Molar mass of O Mass of O2
= 0.247 moles * 16.00 g/mol ≈ 3.95 grams
Therefore, approximately 3.95 grams of oxygen gas is required for the complete combustion of 50 grams of potassium nitrate.
Example 2: Solubility
What is the maximum amount of potassium nitrate that can dissolve in 100 mL of water at 25°C?
Solution:
The solubility of potassium nitrate in water at 25°C is approximately 31 grams per 100 mL of water.
Therefore, the maximum amount of potassium nitrate that can dissolve in 100 mL of water at 25°C is 31 grams.
Frequently Asked Questions on Potassium Nitrate Formula
1: What is special about potassium nitrate?
Answer: Potassium nitrate, also known as saltpeter, has several special properties and uses. Among them, the specialty of potassium nitrate is that it can act as an oxidizing agent.
Potassium nitrate is a powerful oxidizing agent. It can provide oxygen to support combustion and is commonly used in fireworks, explosives, and rocket propellants.
2: What is the use of potassium nitrate?
Answer: The use of potassium nitrate (chemical formula: KNO3) is diverse and spans various industries. Here are some common uses of potassium nitrate:
- Fertilizer: Potassium nitrate is used as a source of nitrogen and potassium in fertilizers. It provides essential nutrients for plant growth, promotes healthy root development, and enhances crop yield.
- Food Preservation: Potassium nitrate is used as a food preservative, particularly in cured meats like bacon, ham, and sausages. It helps inhibit the growth of bacteria and acts as a curing agent, extending the shelf life of these products.
- Gunpowder: Potassium nitrate is a crucial component of gunpowder, also known as black powder. When combined with sulfur and charcoal, it forms a highly combustible mixture used as a propellant in firearms and fireworks.
- Medicinal Use: Historically, potassium nitrate was used in certain medicinal preparations for its diuretic and expectorant properties. However, its medical use has significantly declined due to the availability of more effective treatments.
- Heat Transfer Fluid: Potassium nitrate has a high heat capacity and can be used as a heat transfer fluid in some industrial processes, such as solar power systems and thermal storage applications.
3: What is another name for potassium nitrate?
Answer: Potassium nitrate also called saltpetre or nitre is a white solid soluble in water formed by fractional crystallisation of sodium nitrate and potassium chloride solutions.
4: Why is it called potassium nitrate?
Answer: The term “potassium” refers to the element potassium (K), which is an alkali metal with the atomic number 19. Potassium is represented by the symbol K on the periodic table.
The term “nitrate” refers to the nitrate ion (NO3-), which is a polyatomic ion composed of one nitrogen atom bonded to three oxygen atoms. The nitrate ion carries a negative charge.
5: Why is titration used for potassium nitrate?
Answer: Titration is a commonly used analytical technique in chemistry, and it can be used to determine the concentration or amount of a substance in a solution. In the case of potassium nitrate, titration can be used to determine its concentration or purity.
Here’s how titration can be used for potassium nitrate:
- Acid-base titration: Potassium nitrate is a salt that is neutral in nature. However, if it contains any impurities or acidic/basic contaminants, it can affect its properties. In an acid-base titration, a known concentration of an acid or base is added to a solution of potassium nitrate until the reaction between them is complete. The point at which the reaction is complete is indicated by a change in color or pH, and it helps determine the concentration or purity of potassium nitrate.
- Redox titration: Potassium nitrate can also undergo redox reactions, particularly involving its nitrate (NO3-) and nitrite (NO2-) ions. Redox titration involves the transfer of electrons between the analyte (potassium nitrate) and a titrant (a substance with a known redox potential). By measuring the volume of the titrant required to reach a specific endpoint, the concentration of potassium nitrate can be determined.
By using appropriate indicators or instruments to detect the endpoint of the titration, the concentration or purity of potassium nitrate can be accurately determined.
6: Why is KNO3 potassium nitrate?
Answer: Potassium nitrate is called potassium nitrate because it is composed of potassium (K) ions and nitrate (NO3-) ions. The compound is formed through the combination of the element potassium (K) and the polyatomic ion nitrate (NO3-). In the chemical formula KNO3, the symbol K represents potassium, and the symbols NO3 represent the nitrate ion. The ratio of potassium ions to nitrate ions in the compound is 1:1, resulting in the formula KNO3. This naming convention is based on the rules of chemical nomenclature, which help identify and distinguish different compounds based on their constituent elements and ions.
7: Why is potassium nitrate used in fireworks?
Answer: Potassium nitrate is used in fireworks for its oxidizing properties. It serves as an essential component of the firework composition, commonly known as the “oxidizer” or “oxidizing agent.” When potassium nitrate is heated or ignited, it releases oxygen, which supports the combustion of other compounds in the firework. This oxygen supply helps sustain and accelerate the chemical reactions that produce the vibrant colors, sparks, and explosions characteristic of fireworks. Additionally, potassium ions from potassium nitrate can contribute to the color of the firework display. Overall, potassium nitrate plays a crucial role in fireworks by providing the necessary oxygen and participating in the chemical reactions that create the visual and auditory effects.
8: How is KNO3 used in fertilizer?
Answer: Potassium nitrate (KNO3) is commonly used in fertilizers as a source of potassium and nitrogen, two essential nutrients for plant growth. The high solubility of potassium nitrate allows for easy uptake by plant roots, ensuring efficient nutrient absorption. The potassium component of potassium nitrate helps in overall plant development, including the formation of flowers, fruits, and seeds, as well as improving plant resistance to diseases and stress. The nitrogen component provides plants with the necessary building blocks for proteins, enzymes, and chlorophyll, promoting healthy leaf and stem growth. The controlled release of potassium and nitrogen from potassium nitrate fertilizers supports balanced and sustained plant nutrition, leading to improved crop yield and quality. Additionally, potassium nitrate’s low salt index makes it suitable for sensitive crops and can help prevent salt build-up in the soil.








