Table of Contents
Sodium hypochlorite is a chemical compound with the formula NaOCl. It is a pale yellow-green liquid that is commonly used as a disinfectant and bleaching agent. Sodium hypochlorite is a strong oxidizing agent and has the ability to kill bacteria, viruses, and other microorganisms by oxidizing their cell components. It is widely used for water treatment, both in industrial and domestic applications, as well as for household cleaning and disinfection purposes. Sodium hypochlorite is also utilized in medical settings for sterilizing equipment and surfaces. However, it should be handled with care as it can be corrosive and potentially hazardous if not used properly.
Formula of Sodium Hypochlorite Formula
The chemical formula of sodium hypochlorite is NaOCl.
Sodium hypochlorite is a chemical compound with the formula NaClO. It is commonly known as bleach and is widely used as a disinfectant, sanitizer, and cleaning agent. Let’s break down the formula of sodium hypochlorite to understand its composition.
– Na: The symbol Na represents the element sodium. Sodium is an alkali metal with atomic number 11. It is highly reactive and forms various compounds, including sodium hypochlorite.
– Cl: The symbol Cl represents the element chlorine. Chlorine is a halogen gas with atomic number 17. It is highly reactive and has strong oxidizing properties.
– O: The symbol O represents the element oxygen. Oxygen is a non-metal with atomic number 8. It is essential for various biological and chemical processes.
The formula NaClO indicates that sodium hypochlorite is composed of one sodium atom (Na), one chlorine atom (Cl), and one oxygen atom (O). Sodium is a cation with a +1 charge (Na+), chlorine is an anion with a -1 charge (Cl–), and oxygen is neutral. Therefore, to balance the charges, one sodium atom combines with one hypochlorite ion (ClO–) to form sodium hypochlorite (NaClO).
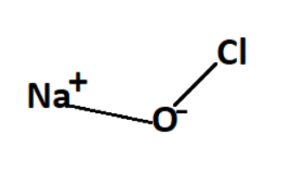
Structure of Sodium Hypochlorite Formula
Sodium hypochlorite consists of a sodium cation (Na+) and a hypochlorite anion (OCl–). The hypochlorite ion is composed of one chlorine atom (Cl) bonded to one oxygen atom (O) through a single covalent bond, and the negative charge is delocalized over the entire hypochlorite ion.
Physical properties of Sodium Hypochlorite Formula
– Appearance: Sodium hypochlorite is typically a pale yellow-green liquid.
– Odor: It has a characteristic chlorine-like odor.
– Density: The density of sodium hypochlorite solution varies depending on the concentration but is typically around 1.1 to 1.2 g/mL.
– Solubility: It is highly soluble in water.
Chemical properties of Sodium Hypochlorite Formula
– Oxidizing Agent: Sodium hypochlorite is a strong oxidizing agent, meaning it can donate oxygen or accept electrons from other substances during chemical reactions.
– Decomposition: It can decompose upon exposure to heat, light, or certain chemicals, releasing oxygen gas and potentially toxic chlorine gas.
– Disinfectant: Sodium hypochlorite is commonly used as a disinfectant and bleaching agent due to its strong oxidizing properties. It can kill bacteria, viruses, and other microorganisms by oxidizing their cell components.
Uses of Sodium Hypochlorite Formula
– Water Treatment: Sodium hypochlorite is used for water disinfection and purification in various applications, such as in swimming pools, drinking water treatment, and wastewater treatment.
– Household Cleaning: It is an ingredient in many household bleach products used for cleaning and disinfecting surfaces, removing stains, and whitening fabrics.
– Medical Applications: Sodium hypochlorite solutions are used in healthcare settings for sterilizing medical equipment and disinfecting surfaces.
– Industrial Applications: It is used in various industrial processes, including pulp and paper bleaching, textile bleaching, and chemical synthesis.
Sodium Hypochlorite Conclusion
sodium hypochlorite has the chemical formula NaClO. It consists of one sodium atom, one chlorine atom, and one oxygen atom. Sodium hypochlorite is widely used for its disinfecting and cleaning properties, and its formula represents the combination of sodium, chlorine, and oxygen in the compound.
Solved examples on Sodium Hypochlorite (NaOCl):
Example 1: A water treatment plant needs to disinfect a water supply contaminated with harmful bacteria. They decide to use sodium hypochlorite for this purpose. The concentration of the sodium hypochlorite solution they have is 5% (w/v). The volume of water to be treated is 10,000 liters. Calculate the amount of sodium hypochlorite solution (in liters) required for the disinfection process.
Solution:
To determine the amount of sodium hypochlorite solution required, we need to consider the desired concentration and volume of the solution.
Given:
Concentration of sodium hypochlorite solution: 5% (w/v)
Volume of water to be treated: 10,000 liters
To disinfect the water, we need to determine the volume of sodium hypochlorite solution needed.
5% (w/v) concentration means that 5 grams of sodium hypochlorite is present in 100 mL (or 0.1 liter) of solution.
Converting the volume of water to be treated to liters:
10,000 liters
Converting the concentration to the amount of sodium hypochlorite solution needed:
(5 grams/100 mL) x (10,000 liters) = 5,000 grams = 5 liters
Therefore, 5 liters of sodium hypochlorite solution are required for disinfecting the water.
Example 2: A person wants to whiten their stained white shirt using sodium hypochlorite bleach. They have a bottle of bleach with a concentration of 3% (w/v) sodium hypochlorite. The person fills a bucket with 5 liters of water and adds the appropriate amount of bleach to achieve a 1:10 bleach-to-water ratio. Calculate the volume of sodium hypochlorite bleach (in milliliters) to be added to the bucket.
Solution:
To calculate the volume of sodium hypochlorite bleach needed, we can use the desired bleach-to-water ratio and the concentration of the bleach solution.
Given:
– Concentration of sodium hypochlorite bleach: 3% (w/v)
– Volume of water in the bucket: 5 liters
– Desired bleach-to-water ratio: 1:10
The desired bleach-to-water ratio is 1 part bleach to 10 parts water. This means that for every 1 part of bleach, there should be 10 parts of water.
Converting the volume of water to milliliters:
5 liters x 1000 mL/L = 5000 mL
To determine the volume of bleach needed, we can set up a ratio:
1 part bleach : 10 parts water
x mL bleach : 5000 mL water
Cross-multiplying and solving for x:
x mL bleach = (1 part bleach / 10 parts water) x 5000 mL water
x mL bleach = 500 mL bleach
Therefore, 500 mL of sodium hypochlorite bleach should be added to the bucket to achieve the desired bleach-to-water ratio.
Frequently asked questions on Sodium Hypochlorite
1: Which formula is used for sodium hypochlorite?
Answer: The formula used for sodium hypochlorite is NaOCl.
2: What is the formula of 1% sodium hypochlorite?
Answer: To determine the formula of a 1% sodium hypochlorite solution, we need to consider the concentration given.
A 1% solution typically refers to a mass/volume percent concentration, which means that 1 gram of sodium hypochlorite is dissolved in 100 milliliters (or 0.1 liter) of solution.
Therefore, the formula for a 1% sodium hypochlorite solution can be expressed as 1g/100mL or 1g/0.1L.
3: What is the strongest sodium hypochlorite solution?
Answer: The strongest commonly available sodium hypochlorite solution is typically around 12-15% (w/v) concentration. This is often referred to as “industrial-strength” sodium hypochlorite. However, it’s important to note that the concentration of sodium hypochlorite can vary depending on the specific product and intended use. Solutions with higher concentrations, such as 20-25%, may be available for specialized applications or commercial/industrial purposes. It’s crucial to handle high-concentration sodium hypochlorite solutions with caution as they can be corrosive and potentially hazardous.
4: What type of bond is sodium hypochlorite?
Answer: Sodium hypochlorite (NaOCl) consists of an ionic bond between the sodium cation (Na+) and the hypochlorite anion (OCl^-). The sodium atom donates one electron to the hypochlorite ion, resulting in the formation of an ionic bond. The oxygen and chlorine atoms within the hypochlorite ion are held together by covalent bonds, sharing electrons to form stable molecular units.
Therefore, the bond in sodium hypochlorite is predominantly ionic, with some covalent character within the hypochlorite ion itself.
5: What is the Common Name of Sodium Hypochlorite?
Answer: The common name of sodium hypochlorite is bleach. Sodium hypochlorite is a common active ingredient in household bleach products, which are used for cleaning, disinfection, and laundry purposes.
6: Is sodium hypochlorite the same as bleach?
Answer: Yes, sodium hypochlorite is commonly known as bleach. Bleach is a solution of sodium hypochlorite in water and is widely used as a disinfectant, cleaning agent, and laundry bleach.
7: Will sodium hypochlorite kill weeds?
Answer: Sodium hypochlorite can be an effective weed killer. It acts as a broad-spectrum herbicide and can be used to control or eliminate unwanted weeds and vegetation. However, it should be used with caution and according to the instructions provided to avoid harming desirable plants or the environment.
8: Is sodium hypochlorite an ionic compound?
Answer: Yes, sodium hypochlorite is an ionic compound. It is composed of positively charged sodium ions (Na+) and negatively charged hypochlorite ions (ClO–). The electrostatic attraction between these ions forms the ionic bond that holds the compound together.
9: Is sodium hypochlorite harmful to humans?
Answer: Sodium hypochlorite can be harmful to humans if used improperly or in high concentrations. It is a corrosive substance and can cause skin and eye irritation, respiratory issues, and digestive problems. Ingesting or inhaling large amounts of sodium hypochlorite can be toxic. It is important to handle and use sodium hypochlorite carefully, following safety guidelines and precautions, such as wearing protective clothing and avoiding direct contact or inhalation.






