Table of Contents
Structure of the Atom Class 9 Extra Questions Science Chapter 4
Extra Questions for Class 9 Science Chapter 4 Structure of the Atom
Structure of the Atom Class 9 Extra Questions Very Short Answer Questions
Question 1.
Which subatomic particle is absent in an ordinary hydrogen atom?
Answer:
Neutron.
Question 2.
J. Chadwick discovered a subatomic particle which has no charge and has mass nearly equal to that of a proton. Name the particle and give its location in the atom.
Answer:
The particle is neutron and it is present in the nucleus of the atom.
Question 3.
Is it possible for the atom of an element to have one electron, one proton and no neutron? If so, name the element. [NCERT Exemplar]
Answer:
Yes, it is true for hydrogen atom which is represented as \(_{1}^{1} \mathrm{H}\).
Question 4.
Electron attributes negative charge, protons attribute positive charge. An atom has both but why there is no charge?
Answer:
The positive and negative charges of protons and electrons are equal in magnitude. So, atom as a whole is electrically neutral.
Question 5.
Write the electronic configuration of an element whose atomic number is 12.
Answer:
K, L, M
2, 8, 2
Question 6.
What do you understand by ground state of an atom?
Answer:
The state of an atom where all the electrons in the atom are in their lowest energy levels is called the ground state.
Question 7.
What is the maximum number of electrons which can be accommodated in ‘N’ shell?
Answer:
N shell can accommodate maximum 32 electrons.
Question 8.
Write the correct representation of an element ‘X’ which contains 15 electrons and sixteen neutrons.
Answer:
The correct representation of the element X is \(_{ 15 }^{ 31 }{ X }\).
Question 9.
What will be the valency of an atom if it contains 3 protons and 4 neutrons?
Answer:
The valency of the atom will be one.
Question 10.
Which of the following pairs are isotopes?
(i) \(_{84}^{209} \mathrm{x},_{84}^{210} \mathrm{x}\)
(ii) \(\begin{array}{c}{232} \\ {90}\end{array} \mathbf{Z}, \begin{array}{c}{231} \\ {91}\end{array} \mathbf{Z}\)
Answer:
\(_{ 84 }^{ 209 }{ X }\) and \(_{ 84 }^{ 210 }{ X }\) are isotopes.
Question 11.
Out of elements \(_{ 17 }^{ 34 }{ X }\) and \(_{ 18 }^{ 40 }{ Y }\), which is chemically more reactive and why?
Answer:
The elements \(_{ 17 }^{ 34 }{ X }\) is more reactive because its outermost shell is incomplete.
Question 12.
One electron is present in the outermost shell of the atom of an element X. What would be the nature and value of charge on the ion formed if this electron is removed from the outermost shell? [NCERT Exemplar]
Answer:
The charge would be +1.
Question 13.
In the atom of an element X, 6 electrons are present in the outermost shell. If it acquires noble gas configuration by accepting requisite number of electrons, then what would be the charge on the ion so formed ? [NCERT Exemplar]
Answer:
– 2.
Question 14.
Give two important applications of radioactive isotopes.
Answer:
- An isotope of carbon-12, C14, is used in carbon dating.
- U235 is used in the nuclear reactors to generate electricity.
Question 15.
Which isotope of hydrogen is present in heavy water?
Answer:
Among the three isotopes of hydrogen, deuterium (\(_{1}^{2} \mathrm{H}\))is found in heavy water.
Question 16.
Chemical formula of a metal sulphate is MSO4. What will be the formula of its chloride?
Answer:
MCl2
Question 17.
An element ‘A’ has valency +3, while another element ‘B’ has valency -2. Give the formula of their compound formed when ‘A’ reacts with ‘B’.
Answer:
Element ‘A’ valency +3 (left)
Element ‘B’ valency – 2 (right)
![]()
Chemical formula = A2B3
Question 18.
Valency of an element X is 3. Write the chemical formula of its oxide.
Answer:
X2O3
Question 19.
Will 35Cl and 37Cl have different valencies? Justify your answer. [NCERT Exemplar]
Answer:
No, 35Cl and 37Cl are isotopes of an element.
Question 20.
The atomic number of calcium and argon are 20 and 18 respectively, but the mass number of both these elements is 40. What is the name given to such a pair of elements? [NCERT Exemplar]
Answer:
Isobars
Structure of the Atom Class 9 Extra Questions Short Answer Questions—I
Question 1.
How do you know that nucleus is very small as compared to the size of atom?
Answer:
Rutherford observed that when a-particles were bombarded on a very thin foil they bounced back. But the number of a-particles bouncing back got doubled when he doubled the thickness of gold foil. Then he concluded that the area of nucleus is very small in comparison to the total area of the atom.
Question 2.
Write two characteristics of the canal rays.
Answer:
- The canal rays are deflected by the magnetic fields in a direction opposite to that of the cathode rays.
- They consist of positively charged particles.
Question 3.
Write the electronic configuration of a positively charged sodium ion (Na+). Atomic number of sodium is 11.
Answer:
Number of electrons in Na atom = Atomic number = 11
Number of electrons in Na+ ion = 11 – 1 = 10
Electronic configuration of Na+ ion: 2, 8
Question 4.
The electronic configuration of phosphorus atom is 2, 8, 5. Give the electronic configuration of P3- ion.
Answer:
Electronic configuration of P = 2, 8, 5
P atom gains 3e– to form P3-
∴ P3- has configuration = 2, 8, (5 + 3) = 2, 8, 8
Question 5.
The atomic number of Al and Cl are 13 and 17, respectively. What will be the number of electrons in Al3+ and Cl–?
Answer:
Atomic number of Al = Number of electrons = 13
Number of electrons in Al3+ = 13 – 3 = 10
Atomic number of chlorine = Number of electrons = 17
Number of electrons in Cl– = 17 + 1 = 18
Question 6.
Write down the electron distribution of chlorine atom. How many electrons are there in the L shell? (Atomic number of chlorine is 17). [NCERT Exemplar]
Answer:
The electronic distribution of Cl is 2, 8, 7. The L shell has eight electrons.
Question 7.
Define valence electrons. Which electrons of an atom are involved in the chemical bond formation with other atoms?
Answer:
The electrons present in the outermost shell of an atom or ion are known as valence electrons.
In a chemical bond formation, only valence electrons of an atom take part.
Question 8.
Why do helium, neon and argon have a zero valency? [NCERT Exemplar]
Answer:
Helium has two electrons in its energy shell, while argon and neon have 8 electrons in their valence shells. As these have maximum number of electrons in their valence shells, they do not have any tendency to combine with other elements. Hence, they have a valency equal to zero.
Question 9.
Helium atom has 2 electrons in its valence shell but its valency is not 2. Explain. [NCERT Exemplar]
Answer:
Helium atom has 2 electrons in its valence shell and its duplet is complete. Hence, the valency is zero.
Question 10.
Find out the valency of the atoms represented by the Figs. (a) and (b) [NCERT Exemplar]
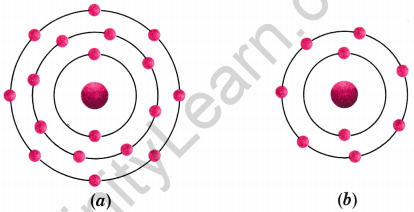
Answer:
(a) 0
(b) 1
Question 11.
Identify the Na+ ion from the following figures. What is the valency of sodium atom? Give reason.
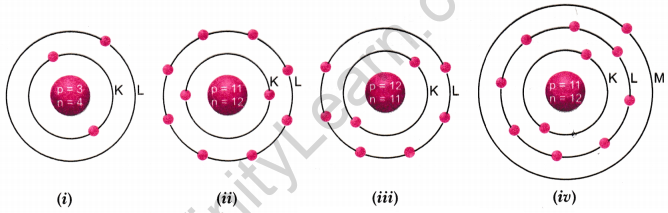
Answer:
Figure number (ii) is correct because sodium ion (Na+) is formed when one electron is lost.
\(\begin{array}{cc}{\mathrm{Na}} & {\longrightarrow \mathrm{Na}^{+}+1 \mathrm{e}^{-}} \\ {(2,8,1)} & {(2,8)}\end{array}\)
The valency of sodium atom is one because stable (octet) electronic configuration is obtained after loss of one electron.
Question 12.
Calculate the number of neutrons present in the nucleus of an element X which is represented as \(_{ 15 }^{ 31 }{ X }\) [NCERT Exemplar]
Answer:
Mass number = No. of protons + No. of neutrons = 31
∴ Number of neutrons = 31 – Number of protons
= 31 – 15 = 16
Question 13.
Why do isotopes show similar chemical properties?
Answer:
Isotopes have same atomic numbers and thus same number of electrons. Therefore, they have the same electronic configuration which provides them similar chemical properties.
Question 14.
An element ‘X’ has a valency 3(+):
(a) Write the formula of its phosphide.
(b) Write the formula of its carbonate.
Answer:
(a) XP
(b) X2 (CO3)3
Question 15.
An element ‘Z’ forms the following compound when it reacts with hydrogen, chlorine, oxygen and phosphorous.
ZH3, ZCl3, Z2O3 and ZP
(a) What is the valency of element ‘Z’?
(6) Element ‘Z’ is metal or non-metal?
Answer:
(a) The valency of ‘Z’ is 3.
(b) Element ‘Z’ is a metal because it is electropositive and is reacting with non-metals.
Structure of the Atom Class 9 Extra Questions Short Answer Questions-II
Question 1.
List any three distinguishing features between the models of an atom proposed by J.J. Thomson and Ernest Rutherford.
Answer:
| J. J. Thomson Model of Atom | Rutherford’s Model |
| 1. Positive charge forms a kernel. | 1. Nucleus (positive charge) is in the centre. |
| 2. Electrons present throughout the atom. | 2. Electrons revolve in orbits. |
| 3. No space is empty. | 3.Most of the space is empty. |
Question 2.
In the gold foil experiment of Geiger and Marsden, that paved the way for Rutherford’s model of an atom, ~ 1.00% of the a-particles were found to deflect at angles > 50°. If one mole of a-particles were bombarded on the gold foil, compute the number of a-particles that would deflect at angles less than 50°. [NCERT Exemplar]
Answer:
% of α-particles deflected more than 50° = 1% of a-particles.
% of α-particles deflected less than 50° = 100 – 1 = 99%
Number of particles that deflected at an angle less than 50°
= \(\frac{99}{100} \times 6.022 \times 10^{23}\)
= \(\frac{596.178}{100} \times 10^{23}\)
= 5.96 × 1023
Question 3.
Predict the valency of the following elements
(i) A (Atomic number 5)
(ii) B (Atomic number 12)
(iii) C (Atomic number 14)
(iv) D (Atomic number 17)
Answer:
(i) Valency of element ‘A’ = 8 – 5 = 3
(ii) Valency of element ‘B’ = 12 – 10 = 2
(iii) Valency of element ‘C’= 14 – 10 = 4
(iv) Valency of element ‘D’= 18 – 17 = 1
Question 4.
An element ‘X’ contains 6 electrons in ‘M’ shell as valence electrons:
(a) What is the atomic number of ‘X’?
(b) Identify whether ‘X’ is a metal or non-metal.
Answer:
(a) If ‘X’ contains 6 electrons in ‘M’ shell as valence electrons, then the electronic configuration of‘X’ is K = 2, L = 8, M = 6
∴ Atomic number = 16
(b) ‘X’ is a non-metal.
Question 5.
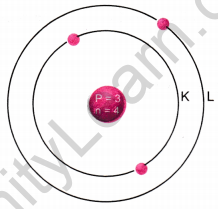
The atomic number of lithium is 3. Its mass number is 7.
(a) How many protons and neutrons are present in a lithium atom?
(b) Draw the diagram of a lithium atom.
Answer:
(a) Number of neutrons = Mass number – atomic number
Number of neutrons = 7 – 3 = 4
Number of protons = atomic number
∴ Number of protons = 3
(b) Structure of a lithium atom
Question 6.
Complete the table on the basis of information available in the symbols given below [NCERT Exemplar]
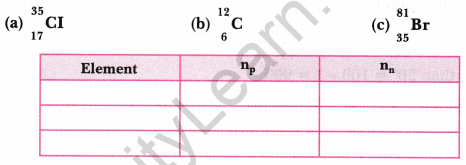
Answer:

Question 7.
In the atom of an element ‘Z’, 5 electrons are present in the outermost shell. It requires noble gas configuration by accepting requisite number of electrons, then what would be the charge on the ion so formed? Write the formula of the compound which will be formed when ‘Z’ reacts with Na atom.
Answer:
Number of electrons in the outermost shell = 5
Number of electrons required to make noble gas configuration = 8 – 5 = 3
The charge on the ion so formed = Z + 3e–
= Z3-
The valency of Z = 3
Chemical formula of the compound: 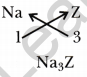
Question 8.
\(_{ 86 }^{ 222 }{ Rn }\) is an isotope of noble gas, radon. How many protons, neutrons and electrons are there in one atom of this radon isotope?
Answer:
Atomic number of radon = 86
The number of protons = 86
The number of electrons = Number of protons
= 86
Number of neutrons = Atomic mass – Atomic number
= 222 – 86 = 136
Question 9.
What information do you get from the figures about the atomic number, valency of atoms X, Y and Z? Give your answer in a tabular form.
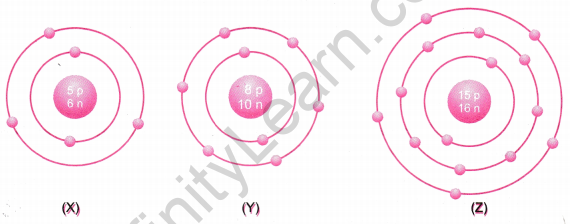
Answer:

Question 10.
Write the molecular formulae for the following compounds:
(a) Copper (II) bromide
(b) Aluminium (III) nitrate
(c) Calcium (II) phosphate
(d) Iron (III) sulphide
(e) Mercury (II) chloride
(f) Magnesium (II) acetate [NCERT Exemplar]
Answer:
(a) CuBr2
(b) Al(NO3)3
(c) Ca3(PO4)2
(d) Fe2S3
(e) HgCl2
(f) Mg(CH3COO)2
Question 11.
Write the molecular formulae of all the compounds that can be formed by the combination of following ions [NCERT Exemplar]
Cu2+, Na+, Fe3+, Cl–, \(\mathrm{so}_{4}^{2-}, \mathrm{PO}_{4}^{3-}\)
Answer:
CuCl2; CuSO4; Cu3 (PO4)2
NaCl; Na2SO4; Na3PO4
FeCl3; Fe2(SO4)3; FePO4
Question 12.
Write the formula of the compounds formed by the following ions.
(a) Mg2+ and S2-
(b) Cu2+ and OH
Name the compounds formed in each case.
Answer:
(a) Ions Mg2+ S2-
Valencies 2 2
Compound: Mg2S2 or MgS; Magnesium sulphate
(b) Ions Cu2+ OH–
Valencies 2 1
Compound: Cu(OH)2; Copper (II) hydroxide.
Structure of the Atom Class 9 Extra Questions Long Answer Questions
Question 1.
(i) State the method of determining the valency of an element if its atomic number is given.
(ii) Determine the valency of the following elements, the atomic numbers of which are given in parenthesis:
Chlorine (17), Sulphur (16), Aluminium (13)
Answer:
(i) The number of electrons gained, lost or shared to make the octet of electrons (in the outermost shell), gives us directly the combining capacity of the element, that is, the valency.

Question 2.
What is the gold foil experiment? Name the scientist who performed this experiment. Write the conclusions and shortcomings of Rutherford’s model of atom.
Answer:
In 1911, Rutherford performed the gold foil experiment. He bombarded a stream of a-particles on a gold foil, a thin sheet which was 0.00006 cm thick in an evacuated chamber. An a-particle is a positively charged helium ion (He2+). A simplified picture of this experiment is shown in the figure.
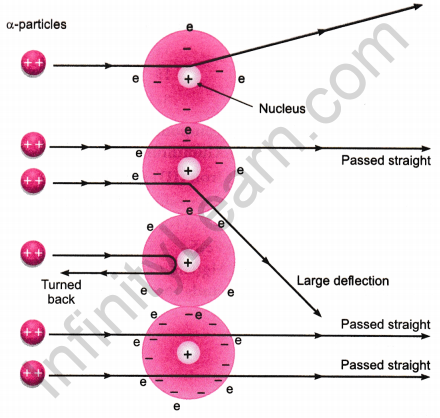
In this famous experiment, the following observations were made.
- Most of the a-particles passed straight through the foil without any deflection. This concluded that most of the space inside of an atom is empty.
- A few α-particles were deflected through small angle and few through larger angles. This happened due to positive charge on a-particles and core (nucleus) of the atom. The heavy positively charged ‘core’ was named as nucleus.
- The number of α-particles which bounced back was very small. This concluded that the volume of the nucleus is very small in comparison to the total volume of the atom.
On the basis of gold foil experiment, Rutherford concluded that an atom consists of nucleus which has positive charge and it is surrounded with electrons which are moving around the nucleus. The number of electrons and protons are equal and the entire mass of the atom is concentrated at its nucleus.
Drawbacks in the Rutherford’s model
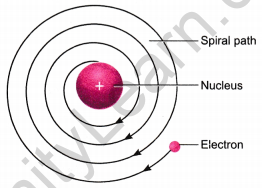
- According to classical electro-magnetic theory, a moving charged particle, such as an electron under the influence of attractive force loses energy continuously in the form of radiations. As a result of this, electron should lose energy and therefore, should move in even smaller orbits ultimately falling into the nucleus. But the collapse does not occur. There is no explanation for this behaviour.
- Rutherford did not specify the number of orbits and the number of electrons in each orbit.
Question 3.
In what way is the Rutherford’s atomic model different from that of Thomson’s atomic model? [NCERT Exemplar]
Answer:
Rutherford proposed a model in which electrons revolve around the nucleus in well-defined orbits. There is a positively charged centre in an atom called the nucleus. He also proposed that the size of the nucleus is very small as compared to the size of the atom and nearly all the mass of an atom is centred in the nucleus. Whereas, Thomson proposed the model of an atom to be similar to a Christmas pudding. The electrons are studded like currants in a positively charged sphere like Christmas pudding and the mass of the atom was supposed to be uniformly distributed.
Question 4.
What are the postulates of Bohr’s model of an atom? [NCERT Exemplar]
Answer:
The postulates put forth by Neils Bohr’s about the model of an atom:
- Only certain special orbits known as discrete orbits of electrons, are allowed inside the atoms.
- While revolving in discrete orbits the electrons do not radiate energy. These orbits are called energy levels. Energy levels in an atom are shown by circles.
These orbits are represented by the letters K, L, M, N, … or the numbers n = 1, 2, 3, 4, ………..
Question 5.
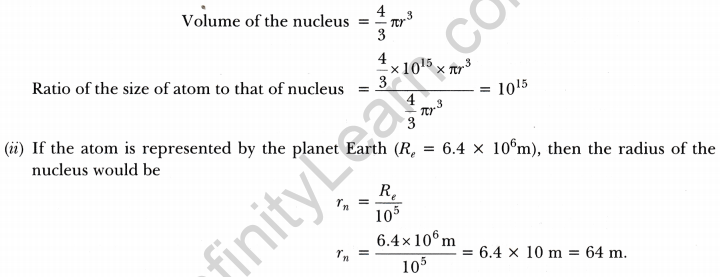
The ratio of the radii of hydrogen atom and its nucleus is ~105. Assuming the atom and the nucleus to be spherical,
(i) what will be the ratio of their sizes?
(ii) If atom is represented by planet Earth ‘Re’ = 6.4 × 106 m. Estimate the size of the nucleus. [NCERT Exemplar]
Answer:
(i) Volume of the sphere = \(\frac{4}{3}\) πr3
Let R be the radius of the atom and r be that of the nucleus.
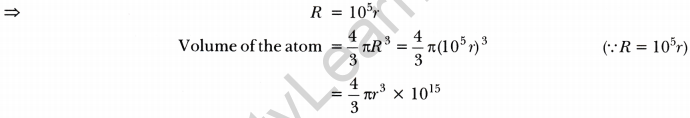
Question 6.
Show diagrammatically the electron distribution in a sodium atom and a sodium ion and also give their atomic number. [NCERT Exemplar] Answer:
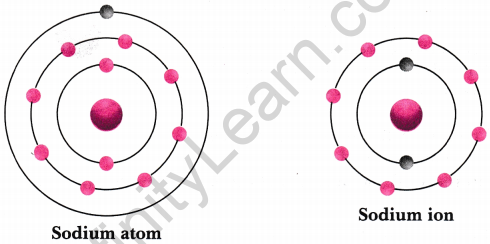
Since the atomic number of sodium atom is 11, it has 11 electrons. A positively charged sodium ion (Na+) is formed by the removal of one electron from a sodium atom. So, a sodium ion has 11 – 1= 10 electrons in it. Thus, electron distribution of sodium ion will be 2, 8. The atomic number of an element is equal to the number of protons in its atom. Since, sodium atom and sodium ion contain the same number of protons, therefore, the atomic number of both is 11.
Question 7.
The given figure depicts the atomic structure of an atom of an element ‘X’.
Write the following information about the element ‘X’.
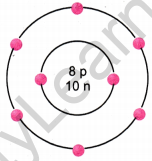
(a) Atomic number of ‘X’
(b) Atomic mass of ‘X’
(c) Valence electrons
(d) Valency of ‘X’
(e) ‘X’ should be metal or non-metal.
Answer:
(a) Atomic number = Number of protons = 8
(ib) Atomic mass = Number of protons + Number of neutrons
= 8 + 10 = 18 u
(c) Valence electrons = 6
(d) Valency of ‘X’ = 8 – 6 = 2
(e) ‘X’ should be non-metal because there are six valence electrons hence it will take two more electrons to complete its outermost shell.
Structure of the Atom Class 9 Extra Questions HOTS (Higher Order Thinking Skills)
Question 1.
One electron is present in the outermost shell of the atom of an element ‘Z’.
(a) What will be the nature of this element?
(b) What will be the value of charge of the ion formed, if this electron is removed from the outermost shell?
Answer:
(a) Element ‘Z’ will be a metal because it has only one electron in the outermost shell, so it is electropositive.
(b) After loss of one electron, ‘Z’ will acquire one positive charge.
Z → Z+ + 1 e–
Question 2.
Composition of the nuclei of two atomic species ‘X’ and ‘Y’ are given below:
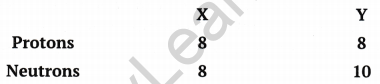
Give the mass number of ‘X’ and ‘Y’. What is the relationship between the two species?
Answer:
(i) Atomic mass of element ‘X’ = Number of protons + Number of neutrons
= 8 + 8 = 16 u
(ii) Atomic mass of element ‘Y’ = Number of neutrons + Number of protons
= 10 + 8 = 18 u
Relationship between X and Y: The atomic number of both the elements is same but their atomic masses are different. Hence,they are isotopes of each other.
Question 3.
An atom ‘M’ of an element reacts with oxygen to form M2O3. Calculate the valency of the element ‘M’.
Answer:
Two atoms of element ‘M’ combine with 3 atoms of oxygen.
∴ Number of oxygen atoms combining with one atom of element ‘M’ = \(\frac{3}{2}\)
Therefore, the valency of element ‘M’ = \(\frac{3}{2}\) × 2 = 3
Question 4.
Complete the following gaps in the given table:
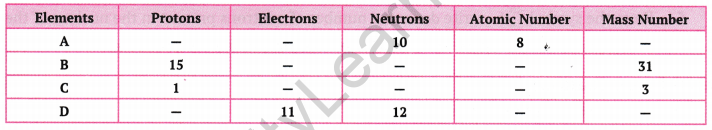
Answer:
We know that the number of protons = Atomic number
Number of protons = Number of electrons
Mass number = Number of protons + number of neutrons
Using these relationships, we can fill up these gaps as follows:
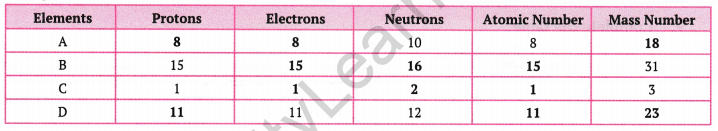
Question 5.
Explain why chlorine, whether as the element or its compounds, always has relative atomic mass of about 35.5.
Answer:
The relative atomic mass is the average mass of one of the atoms and has to take into account the relative abundances of the various isotopes.
Natural chlorine always contains about \(\frac{3}{4} \times_{17}^{35} \mathrm{Cl} \text { and } \frac{1}{4} \times_{17}^{37} \mathrm{Cl}\).
Therefore, relative atomic mass of chlorine = \(\frac{3}{4} \times 35+\frac{1}{4} \times 37\)
= 35.5 u
Question 6.
An element ‘X’ has mass number 4 and atomic number 2. Write the valency of this element. Will it react with other atoms of different elements? [NCERT Exemplar]
Answer:
We know that only valence electrons take part in bond formation with different atoms. In the atom of ‘X’ element there are only two electrons since atomic number is 2. Thus, K shell is fully filled for this atom. Hence, its valency is zero. It will not react with other atoms of different elements.
Question 7.
How many electrons will weigh 1 g?
Answer:
Mass of an electron = 9.11 × 10-31 kg
∴ Mass of 9.11 × 10-31 kg = 1 electron
Now, mass of 1g, i.e., 10-31 kg will have \(\frac{1}{9.11 \times 10^{-31}}\) × 10-3 electrons
= 1.098 × 1027 electrons.









