Table of Contents
The Class 8 Science Chapter 14 Solutions have been simplified to make it easier to grasp all of the chapter’s concepts and subjects. You can now check over the concise explanations provided in these NCERT Exemplar Solutions for Class 8 Science to gain a better understanding of the various ideas covered in the chapter Chemical Effects of Electric Current. Leave your exam anxieties behind and use these tutorials to easily grasp challenging ideas in Chemical Effects of Electric Current. Now go to Infinity Learn’s main website and look at the Class 8 Science Chapter 14 question and answer PDF. Infinity Learn is a website that offers students free NCERT Exemplar Solutions and other study tools. Maths Students looking for better solutions can download Class 8 Maths NCERT Exemplar Solutions to help them review the entire syllabus and improve their grades.
Have you been having trouble finding dependable study materials to help you prepare for your upcoming exams? INFINITY LEARN has made it easy for you to understand key concepts in Chemical Effects of Electric Current by providing free NCERT Exemplar Solutions for Class 8 Chapter 14 in PDF format, which includes all themes and subtopics as well as related question banks.
NCERT Exemplar Solutions for Class 8 Science Chapter 14 – Chemical Effects of Electric Current
NCERT Exemplar Solutions for Class 8 Chapter 14: Key Features
- A few of the most important characteristics of NCERT answers for class 8 Chemical Effects of Electric Current are listed here.
- Acquire a thorough understanding of the various impacts of electric current.
- Recognize the various safety precautions to consider when undertaking operations that require transferring electricity through an object.
Access Answers to NCERT Exemplar Solutions for Class 8 Science Chapter 14 – Chemicals Effects of Electric Current
Multiple-choice Questions
- An electric current can produce
(a) heating effect only.
(b) chemical effect only.
(c) magnetic effect only.
(d) chemical, heating, and magnetic effects.
Answer – (d) chemical, heating, and magnetic effects.
Explanation:
- Electric current induces various effects when passing through different mediums:
- In a conducting solution, electric current triggers a chemical reaction, demonstrating the chemical effect of electric current.
- When electricity flows through a bulb, the filament heats up, causing the bulb to emit light, illustrating the heating effect of electric current.
- Passing an electric current through a circuit generates a magnetic field around it, showcasing the magnetic effect of electric current.
- Boojho and Paheli performed experiments taking similar bulbs and cells but two different solutions, A and B, as shown in Fig.14.1.
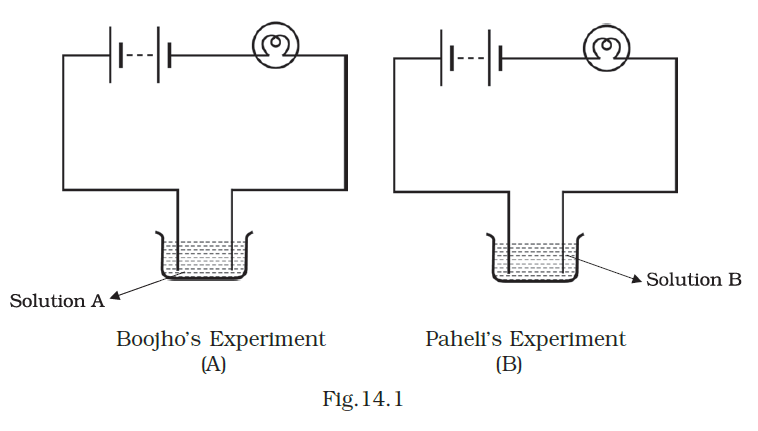
They found that the bulb in setup A glows more brightly as compared to that of setup B. You would conclude that
(a) higher current is flowing through the circuit in setup A.
(b) higher current is flowing through the circuit in setup B.
(c) equal current is flowing through both the circuits.
(d) the current flowing through the circuits in the two setups cannot be compared in this manner.
Answer – (a) higher current is flowing through the circuit in setup A.
Explanation:
Setup A exhibits a higher current flow within the circuit due to the superior conductivity of solution A in comparison to solution B.
| More Resources – NCERT Solutions for Class 8 | |
| NCERT Solutions for Class 8 Science | NCERT Solutions for Class 8 Social Science |
| NCERT Solutions for Class 8 English | NCERT Solutions for Class 8 Maths |
- Boojho’s uncle has set up an electroplating factory near his village. He should dispose of the waste of the factory
(a) in the nearby river.
(b) in the nearby pond.
(c) in the nearby cornfield.
(d) according to the disposal guidelines of the local authority.
Answer – (d) according to the disposal guidelines of the local authority.
Explanation:
Proper waste disposal is crucial in the electroplating industry to prevent water pollution and the release of hazardous chemicals into water sources. It is essential to adhere to the guidelines provided by the local authority for waste disposal.
- When an electric current is passed through a conducting solution, there is a change of colour of the solution. This indicates
(a) the chemical effect of current.
(b) the heating effect of current.
(c) the magnetic effect of current.
(d) the lightning effect of current.
Answer – (a) the chemical effect of current
Explanation:
Passing an electric current through a solution induces a chemical reaction, leading to a change in color. This phenomenon exemplifies the chemical effect of electric current.
- Which one of the following solutions will not conduct electricity?
(a) lemon juice
(b) vinegar
(c) tap water
(d) vegetable oil
Answer –
Answer – (d) vegetable oil
Explanation:
The absence of ions in vegetable oil prevents it from conducting electricity.
- Which of the following metals is used in electroplating to make objects appear shining?
(a) iron
(b) copper
(c) chromium
(d) aluminium
Answer – (c) chromium
Explanation:
Chromium metal is utilized in electroplating to impart a shiny appearance to objects due to its inherent luster and scratch-resistant properties.
- Which of the following solutions will not make the bulb in Fig 14.2 glow?
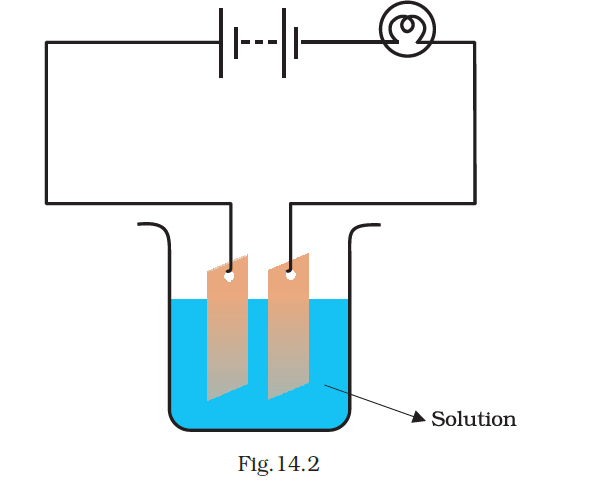
(a) sodium chlorides
(b) copper sulphate
(c) silver nitrate
(d) sugar solution in distilled water
Answer – (d) sugar solution in diluted water
Explanation:
A sugar solution is neither acidic nor basic and lacks the ability to ionize for electricity conduction. Similarly, distilled water contains no ions for electrical conductivity. Therefore, the combination of sugar solution and distilled water will not conduct electricity.
Very Short Answer Questions
- Fill in the blanks
(a) The object to be electroplated is taken as ____________ electrode.
(b) One of the most common applications of chemical effect of electric current is ______________.
(c) Small amount of a mineral salt present naturally in water makes it a______________ of electricity.
(d) Electroplating of ______________ is done on objects like water taps and cycle bell to give them a shiny appearance.
Answer –
(a) Cathode
(b) Electroplating
(c) Conductor
(d) Chromium
- Why is a layer of zinc-coated over iron?
Answer – Iron easily reacts with atmospheric oxygen to produce rust. To inhibit rust formation, a protective zinc coating is applied over the iron surface.
- Will the solution of sugar in distilled water conduct electricity?
Answer – Correct, the sugar solution is neutral, neither acidic nor basic, and therefore does not ionize to facilitate the conduction of electricity.
- Name the effect of current responsible for the glow of the bulb in an electric circuit.
Answer –
Heating effect of electric current.
Short Answer Questions
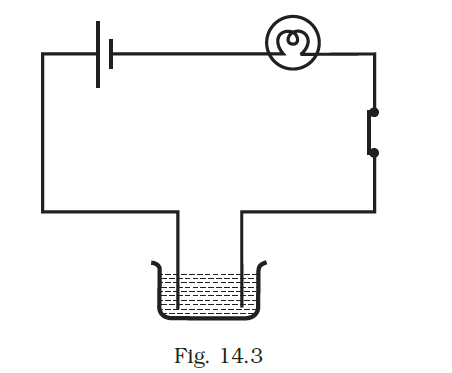
- Boojho made the circuit given in Fig. 14.3 and observed that the bulb did not glow. On Paheli’s suggestion, he added one more cell in the circuit. The bulb now glows. Explain.
Answer –
Upon adding another cell, the current passing through the bulb increases adequately to illuminate it.
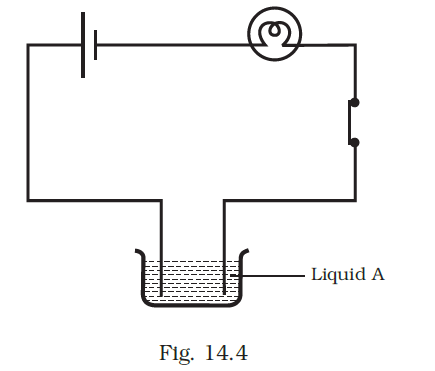
- Paheli set up an experiment using liquid A in the beaker as shown in Fig. 14.4. She observed that the bulb glows. Then she replaced the liquid A by another liquid B. This time the bulb did not glow. Boojho suggested replacing the bulb by an LED. They observed that the LED glows. Explain.
Answer –
The current passing through liquid B might be insufficient to light up the bulb, although it was sufficient to illuminate the LED.
- Paheli wants to deposit silver on an iron spoon. She took a silver nitrate (AgNO3) solution in a beaker and set up a simple circuit for electroplating. Which terminal of the battery should the spoon be connected to? What material should the other electrode be made of?
Answer –
The spoon needs to be linked to the negative terminal of the battery, while the second electrode should be composed of silver.
- Why is tin electroplated on iron to make cans used for storing food?
Answer –
Tin, being less reactive than iron, is applied as a coating over iron to prevent the iron from reacting with food when used for food storage.
- Observe Fig. 14.5.
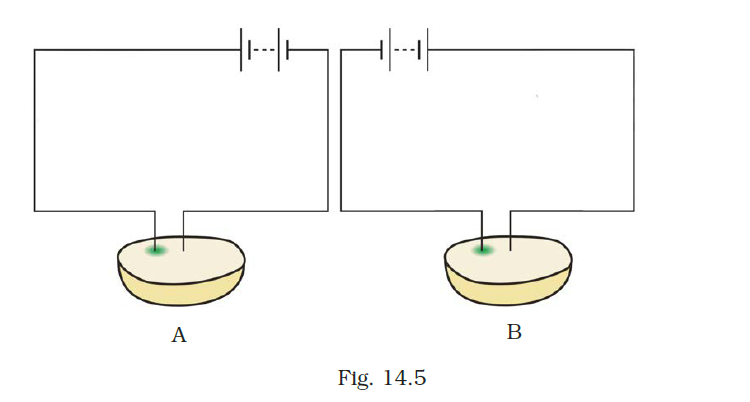
Which of these two circuits A or B shows the correct observation?
Answer –
Diagram A shows the correct observation
- Observe the following circuits carefully. In which circuit will the bulb glow? Write ‘Yes’ or ‘No’ in the blank space provided along each of the circuit given in Fig. 14.6.
Answer –
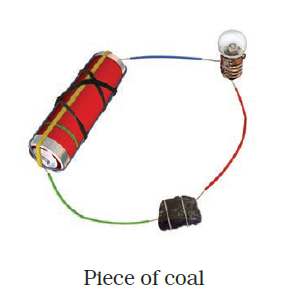 NO
NO
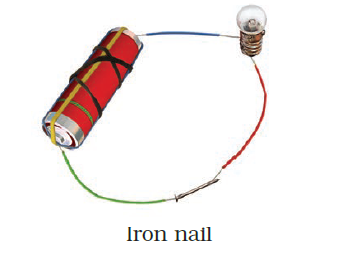
Yes
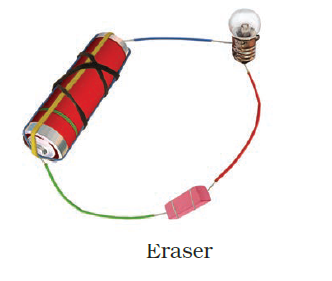
No

Yes
Long Answer Questions
- An electric current is passed through a conducting solution. List any three possible observations.
Answer –
Three possible outcomes are:
- Formation of gas bubbles on the electrodes
- Deposition of metal on the electrodes
- Change in the solution’s color
- Heating of the solution
- In the circuit given as Fig. 14.7, Boojho observed that copper is deposited on the electrode connected to the negative terminal of the battery. Paheli tried to repeat the same experiment. But she could find only one copper plate. Therefore she took a carbon rod as a negative electrode. Will copper be still deposited on the carbon rod? Explain your answer.
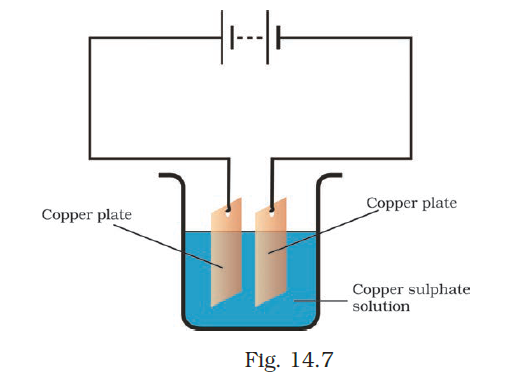
Answer –
Copper ions from the copper sulfate solution will be plated onto the carbon rod. When an electric current passes through the copper sulfate solution, it dissociates into copper and sulfate ions. The copper ions are attracted to the electrode connected to the negative terminal of the battery, which is the carbon rod, where they are deposited. Consequently, Paheli will achieve a copper coating on the carbon rod.
- Observe the circuit given in Fig. 14.8.
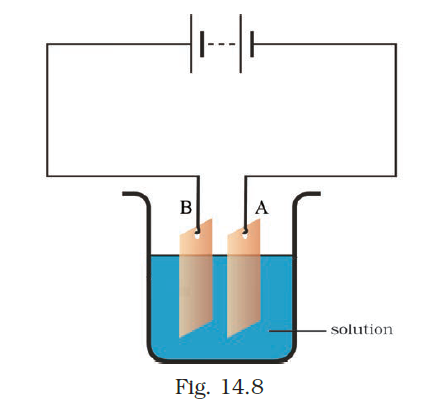
Boojho set up this circuit for purification of copper. What will be the nature of – (i) plate A (ii) plate B (iii) the solution? Explain the process of purification.
Answer –
- i) In plate A- Pure copper
- ii) In plate B- Impure copper
iii) The solution – Copper sulphate solution
The purification process employed here is electroplating.
To perform this process, gather copper sulfate, two copper plates of approximately 10 cm × 4 cm in size, and 250 mL of distilled water. Prepare a clean and dry beaker, then dissolve two teaspoons of copper sulfate in the distilled water. Add a few drops of dilute sulfuric acid to the copper sulfate solution to enhance its conductivity. Clean the copper plates using sandpaper, rinse them with water, and dry them. Connect the copper plates to the terminals of a battery and immerse them in the copper sulfate solution.
When an electric current is passed through the copper sulfate solution, it dissociates into copper and sulfate ions. The free copper ions are attracted to the electrode connected to the negative terminal of the battery and are deposited on it.
- Observe the following circuit given in Fig. 14.9.
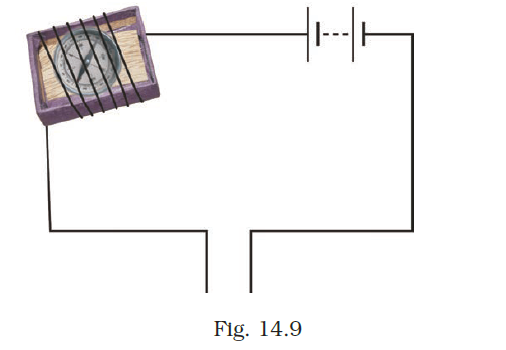
Current does not flow in the circuit if there is a gap between the two wires. Does it indicate that air is a poor conductor of electricity? Does air never conduct electricity? Explain.
Answer –
Typically, air is a poor conductor of electricity. However, under specific circumstances such as lightning, air can conduct electricity.
- Boojho made the circuit shown in Fig. 14.10. He wanted to observe what happens when an electric current is passed through water. But he forgot to add a few drops of lemon juice to water. Will it make any difference to his observations? Explain.
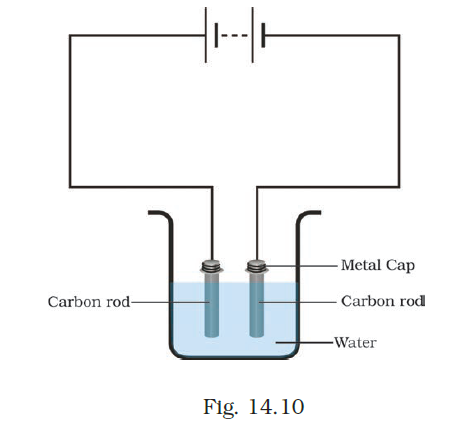
Answer –
In the case of distilled water, without the addition of lemon juice, it remains non-conductive, and the current will not flow through the circuit. Conversely, when the water is saline, a weak current will traverse the circuit, accompanied by the formation of bubbles on the negative electrode.
- Observing that the bulb does not glow in the circuit shown in Fig. 14.11 A, Boojho changed the circuit as shown in Fig 14.11 B. He observed deflection in the magnetic compass.
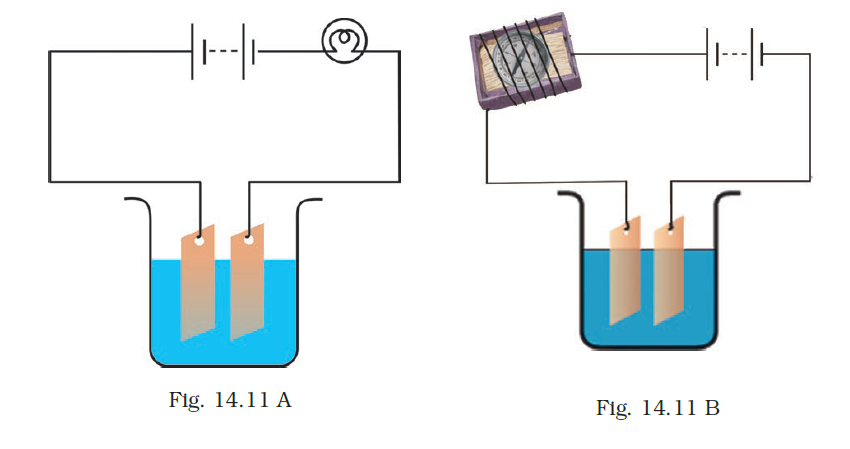
(i)What does the deflection in the magnetic compass indicate?
(ii) Why did the bulb not glow in Fig14.11 A?
(iii) What would be the effect of an increase in the number of turns in the coil wound around the magnetic compass in Fig. 14.11B?
(iv) What will be observed if the number of cells is increased in the circuit shown in Fig. 14.11B?
Answer –
(i) It signifies the existence of current in the circuit.
(ii) The bulb failed to illuminate due to insufficient current for glowing.
(iii) The magnetic compass deflection will intensify.
(iv) The compass deflection will further increase.
- You are provided with a magnetic compass, an empty matchbox, a battery of two cells and connecting wires. Using these objects, how will you make a tester for testing an electric circuit? Draw the necessary circuit diagram and explain.
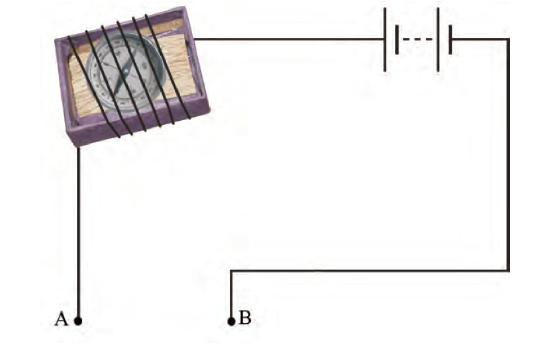
Answer – The deflection of a magnetic compass needle occurs when current passes through the circuit, demonstrating the magnetic effect of the current.
Class 8 Science Solutions Chapter 14 FAQs
Is Infinity Learn providing Solutions for Class 8 Science Chapter 14?
Yes, the Infinity Learn website comes with accurate and detailed solutions for all questions provided in the NCERT Textbook. Infinity Learn brings you NCERT Exemplar Solutions for Class 8 Science, made by our subject matter experts for a smooth and easy understanding of concepts. These solutions come with detailed step-by-step explanations of problems given in the NCERT Textbook. The NCERT Exemplar Solutions of this chapter can be downloaded in the form of a PDF and it can be used as a quick revision tool.
What are the Benefits of Downloading Infinity Learn’s Class 8 Science Chapter 14 Solution?
There are numerous advantages to obtaining Infinity Learn’s NCERT Exemplar Solutions for class 8 science chapter 14 pdf. If you want to learn more about those advantages, see the list of advantages for downloading class 8th science chapter 14 solutions below: Students can sign up for live online classes. Students can memorize all of the answers in order to improve their final science exam scores. All of the responses are 100 percent accurate and dependable. The most brilliant and experienced subject matter experts in India write the answers. Students can access academic help 24 hours a day, seven days a week.
Why Opt for Infinity Learn?
In this chapter, Infinity Learn provides videos, notes, NCERT textbook solutions, other practice book solutions, and assignments that help you learn the concepts and to memorize the concepts for entrance exams and board exams. Most important, Infinity Learn to provides instant doubt support by subject experts.









