Table of Contents
Introduction
Vinegar is a common household product that is primarily composed of acetic acid. Its chemical formula is CH3COOH. The formula CH3COOH represents the structural arrangement of atoms in acetic acid. It consists of two carbon (C) atoms, four hydrogen (H) atoms, and two oxygen (O) atoms. The “CH3” part represents a methyl group (one carbon atom bonded to three hydrogen atoms),
The “COOH” part represents a carboxyl group (one carbon atom double bonded to one oxygen atom and single bonded to another oxygen atom, which is also bonded to a hydrogen atom). In simple terms, the formula CH3COOH signifies that vinegar is composed of carbon, hydrogen, and oxygen atoms arranged in a specific molecular structure.
It is important to note that vinegar may also contain other substances, such as water and trace amounts of impurities, depending on its production method and source. However, the primary component and the defining characteristic of vinegar is acetic acid, which is represented by the chemical formula CH3COOH.
Uses of Vinegar
Vinegar, typically consisting of acetic acid diluted in water, has various uses in different areas. In culinary applications, it is commonly used as a condiment, flavor enhancer, and preservative for food. It can be used in dressings, marinades, pickling, and as a flavoring agent in cooking. Vinegar also finds applications in household cleaning due to its acidity and antimicrobial properties. It can be used as a natural cleaner for surfaces, windows, and even removing stains. Additionally, vinegar has been used for personal care purposes such as hair rinses, skin toners, and foot soaks. It is also utilized in certain industrial processes, such as manufacturing chemicals and pharmaceuticals.
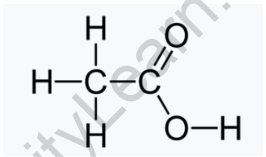
The structure of vinegar is composed of one methyl group and one carboxylic group. Both these groups are bonded with the single sigma bond. In the methyl group, three hydrogen atoms are bonded with the carbon by three-sigma covalent bonds. In the carboxyl group, one hydroxyl group and one oxo group are present. The hydroxyl group is bonded with the carbon by a single sigma bond while the oxo group is bonded with the double bond, one is pi-bond while the other one is the sigma.
Physical Properties of Vinegar
- Appearance: Vinegar is a clear, colorless liquid. It may have a slightly yellowish tint depending on the specific type and production process.
- Odour: Vinegar has a pungent, sour odor that is characteristic of acetic acid.
- Taste: Vinegar has a sour taste due to the presence of acetic acid. The intensity of the sourness can vary depending on the concentration of acetic acid in the vinegar.
- pH: Vinegar is acidic in nature and has a pH typically ranging from 2 to 3. The pH value can vary slightly depending on the specific type and concentration of vinegar.
- Boiling Point: The boiling point of vinegar is around 100 degrees Celsius (212 degrees Fahrenheit). However, the exact boiling point may vary depending on the concentration of acetic acid and other impurities present.
- Freezing Point: Vinegar freezes at approximately 16 degrees Celsius (61 degrees Fahrenheit) or slightly below. The freezing point can be affected by the concentration of acetic acid.
Chemical Properties on Vinegar
- Acidic Nature: Vinegar is an acid due to the presence of acetic acid. It exhibits typical properties of acids, such as the ability to donate protons (H+) in chemical reactions.
- Reactivity with Bases: Vinegar reacts with bases to undergo neutralization reactions. When vinegar is mixed with a base, such as sodium hydroxide (NaOH), it forms a salt (sodium acetate) and water.
CH3COOH + NaOH → CH3COONa + H2O
- Oxidation and Reduction Reactions: Acetic acid in vinegar can participate in oxidation and reduction reactions. For example, acetic acid can be oxidized to produce carbon dioxide and water in the presence of certain oxidizing agents.
- Esterification: Acetic acid in vinegar can undergo esterification reactions. When reacted with an alcohol, such as ethanol, it forms esters (e.g., ethyl acetate) and water.
CH3COOH + C2H5OH → CH3COOC2H5 + H2O
- Reaction with Metals: Acetic acid can react with certain metals, such as zinc (Zn), to produce hydrogen gas and a corresponding metal salt.
2CH3COOH + Zn → Zn(CH3COO)2 + H2
pH-Dependent Reactions: The acidic nature of vinegar (acetic acid) makes it reactive with substances sensitive to pH changes. For example, it can be used as a cleaning agent to remove mineral deposits due to its ability to dissolve calcium carbonate.
Solved Examples on Vinegar Formula
Example 1: A bottle of vinegar contains 500 mL of a solution with a concentration of 6% (v/v) acetic acid. How many milliliters of acetic acid are present in the vinegar?
Solution: Concentration of acetic acid = 6% (v/v) Volume of vinegar = 500 mL
To find the amount of acetic acid, we can use the equation: Amount of acetic acid = Volume of vinegar × Concentration of acetic acid
Amount of acetic acid = 500 mL × 6% = 30 mL
Therefore, there are 30 mL of acetic acid present in the vinegar.
Example 2: When 50 mL of vinegar (5% acetic acid) reacts with excess sodium bicarbonate, how many milliliters of carbon dioxide gas will be produced at standard temperature and pressure?
Solution: To solve this problem, we need to know the balanced chemical equation for the reaction between vinegar (acetic acid) and sodium bicarbonate:
CH3COOH + NaHCO3 → CO2 + H2O + CH3COONa
From the balanced equation, we can see that one mole of acetic acid (CH3COOH) reacts to produce one mole of carbon dioxide (CO2). The molar volume of a gas at standard temperature and pressure (STP) is approximately 22.4 L/mol.
Given: Volume of vinegar (acetic acid) = 50 mL Concentration of acetic acid = 5% (v/v)
To find the amount of acetic acid in moles, we can use the equation: Amount of acetic acid (in moles) = Volume of vinegar (in liters) × Concentration (in moles per liter)
Amount of acetic acid = 50 mL × (5/1000) L/mL = 0.25 moles
Since the stoichiometry of the reaction is 1:1 between acetic acid and carbon dioxide, the amount of carbon dioxide produced will also be 0.25 moles.
Now, we can calculate the volume of carbon dioxide produced at STP:
Volume of carbon dioxide = Amount of carbon dioxide (in moles) × Molar volume at STP
Volume of carbon dioxide = 0.25 moles × 22.4 L/mol = 5.6 L
Therefore, when 50 mL of vinegar (5% acetic acid) reacts with excess sodium bicarbonate, approximately 5.6 liters of carbon dioxide gas will be produced at standard temperature and pressure.
Read More Formulas:
| Magnesium nitride formula | Oxalate formula |
| Sodium citrate formula | Sodium nitrite formula |
| Sulfur dioxide formula | Calcium acetate formula |
| Chlorate formula | Chromate formula |
| Dichromate formula | Potassium phosphate formula |
| Temperature Conversion Formula | Celsius Formula |
Frequently Asked Questions on Vinegar Formula
What are the two chemicals present in vinegar?
The two main chemicals present in vinegar are water (H2O) and acetic acid (CH3COOH). Water makes up the majority of the composition of vinegar, while acetic acid is the compound responsible for its sour taste and pungent smell.
What type of acid is found in vinegar?
The acid found in vinegar is called acetic acid. It is a weak acid with the chemical formula CH3COOH. Acetic acid is responsible for the sour taste and pungent odour of vinegar.
What is the chemical nature of vinegar?
Vinegar is an acidic solution with a chemical nature that is primarily determined by the presence of acetic acid. Acetic acid is a weak acid and is responsible for the characteristic properties of vinegar. It is a polar molecule, which means it has a slightly positive and negative charge at different ends of the molecule. This polarity allows acetic acid to dissolve in water and exhibit acidic properties. Additionally, vinegar may contain other minor components such as water, trace amounts of other acids, and flavour compounds depending on its specific type and production process.
What is the pH of vinegar?
The pH of vinegar can vary depending on the specific type and concentration. Generally, vinegar has a pH level ranging from 2 to 3. This acidity is primarily due to the presence of acetic acid. Vinegar is considered an acidic substance, with a pH lower than 7. The pH scale ranges from 0 to 14, where values below 7 indicate acidity, 7 is neutral, and values above 7 indicate alkalinity.
What is another name for vinegar?
The trivial name acetic acid is the most used and preferred IUPAC name. The systematic name ethanoic acid, a valid IUPAC name, is constructed according to the substitutive nomenclature. The name acetic acid derives from the Latin word for vinegar, acetum, which is related to the word acid itself.









