Oxalate formula
Introduction
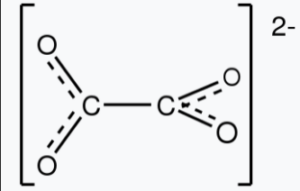
Oxalate compounds are a family of chemical substances that consist of the oxalate ion (C2O42-) combined with different cations. The oxalate ion is a polyatomic ion composed of two carbon atoms (C) and four oxygen atoms (O), arranged in a specific structure.
The chemical formula for oxalate can vary depending on the specific compound. For example, potassium oxalate, the most well-known oxalate compound, has the formula K2C2O4. In this compound, two potassium ions (K+) are combined with one oxalate ion.
Oxalate compounds are commonly found in nature, and they can also be synthesized in the laboratory. They are present in certain plants, such as spinach and rhubarb, as well as in some food products. Oxalates have various applications in industries such as pharmaceuticals, photography, and textile dyeing.
Structure of Oxalate
The formula for oxalate is C2O42-. Oxalate is an anion, and it typically forms compounds by combining with metal ions to achieve charge neutrality. Oxalate refers to a group of chemical compounds that contain the oxalate ion (C2O42-). The chemical formula for oxalate varies depending on the specific compound, as it can combine with different cations to form various salts. However, the simplest and most common form of oxalate is the potassium oxalate compound, with the chemical formula K2C2O4.
The oxalate ion (C2O42-) consists of two carbon atoms (C) bonded to each other through a double bond, and each carbon atom is also bonded to two oxygen atoms (O). The overall charge of the ion is -2.
Physical properties of Oxalate
Oxalate is a colorless, crystalline solid. It is soluble in water and has a melting point of around 189°C. It can form hydrates with water molecules.
Chemical properties of Oxalate
Oxalate is a weak acid and can donate hydrogen ions (H+) in solution. It can also act as a reducing agent, undergoing oxidation-reduction reactions. Oxalate can form insoluble salts with certain metal ions, such as calcium oxalate.
Uses of Oxalate
Oxalate and its derivatives have various applications, including:
- Analytical chemistry: Oxalate is used as a standard substance in chemical analysis and titrations.
- Metal cleaning and polishing: Oxalate compounds are used in cleaning and polishing metal surfaces, such as stainless steel and aluminum.
- Photography: Oxalates are used in some photographic processes as reducing agents and stabilizers.
- Chelating agent: Oxalate can form complexes with metal ions, and it is used as a chelating agent in certain industrial processes and water treatment.
- Chemical synthesis: Oxalate compounds are used as reagents or catalysts in various chemical reactions and organic synthesis.
Oxalate Conclusion
One significant property of oxalate compounds is their ability to form insoluble precipitates with many metal ions. This property makes them useful as chelating agents, capable of binding and removing metal ions from solutions.
It is important to note that while oxalate compounds have some industrial and scientific applications, they should be handled with caution. Some oxalate compounds, such as calcium oxalate, can be toxic and may cause health issues if ingested or inhaled in large amounts.
In summary, oxalate compounds consist of the oxalate ion (C2O42-) combined with different cations. They have various applications in industries and are found in certain plants and food products. Oxalates can form insoluble precipitates with metal ions and have the ability to act as chelating agents. Care should be taken when handling oxalate compounds, as some can be toxic.
Solved examples on oxalate:
Example1: What is the chemical formula of calcium oxalate, a common component of kidney stones?
Answer: The chemical formula of calcium oxalate is CaC2O4. In this compound, one calcium ion (Ca2+) is bonded to one oxalate ion (C2O42-).
Example 2: When oxalate reacts with sulfuric acid, what is the product formed?
Answer: When oxalate (C2O42-) reacts with sulfuric acid (H2SO4), the product formed is carbon dioxide (CO2) and water (H2O). The balanced chemical equation for this reaction is:
H2C2O4 (oxalate) + H2SO4 (sulfuric acid) → CO2 (carbon dioxide) + H2O (water) + SO2 (sulfur dioxide)
Frequently asked questions on Oxalate
1: What are the problems with oxalates?
Answer: Oxalates can pose some potential problems in the following ways:
– Kidney stone formation: High levels of oxalate in the urine can lead to the formation of calcium oxalate kidney stones. These stones can cause pain and discomfort and may require medical intervention for their removal.
– Interference with nutrient absorption: Oxalates can bind with certain minerals, such as calcium and iron, forming insoluble complexes. This can interfere with the absorption of these minerals in the digestive system, potentially affecting their bioavailability.
– Impact on certain health conditions: For individuals with specific health conditions, such as kidney disease or certain gastrointestinal disorders, the presence of high levels of oxalate in the diet may exacerbate their symptoms or worsen their condition.
– Limited food choices: Some individuals may need to follow a low-oxalate diet to manage certain health conditions or minimize the risk of kidney stone formation. This can restrict their food choices and require careful planning to ensure a balanced and varied diet.
2: What foods are highest in oxalates?
Answer: Foods that are highest in oxalates include:
– Spinach: Spinach is one of the most oxalate-rich foods. Other leafy greens like kale and beet greens also contain significant amounts of oxalates.
– Rhubarb: Rhubarb is another high-oxalate food, especially in its raw form. Cooking rhubarb can reduce its oxalate content.
– Beets: Beets, particularly beet greens, are high in oxalates. The roots of beets contain a moderate amount of oxalates.
– Swiss chard: Swiss chard is a leafy green vegetable that contains high levels of oxalates.
– Nuts and seeds: Some nuts and seeds, such as almonds, cashews, peanuts, and sesame seeds, have moderate to high oxalate content.
– Soy products: Certain soy-based foods, including tofu and soy milk, contain moderate levels of oxalates.
– Chocolate and cocoa: Dark chocolate and cocoa powder are sources of oxalates, although the oxalate content can vary.
3: What are the health hazards of oxalate?
Answer: While oxalates are naturally occurring compounds found in many foods, excessive intake or certain health conditions can lead to health hazards. Some potential health hazards associated with oxalates include:
– Kidney stone formation: High levels of oxalate in the urine can increase the risk of calcium oxalate kidney stone formation. These stones can cause severe pain and discomfort and may require medical intervention for their removal.
– Nutrient absorption interference: Oxalates can bind to certain minerals, such as calcium and iron, forming insoluble compounds. This can inhibit the absorption of these minerals in the digestive system, potentially leading to deficiencies if dietary intake is inadequate.
– Gastrointestinal issues: In some individuals, consuming foods high in oxalates may contribute to gastrointestinal issues such as bloating, gas, diarrhea, or constipation. These symptoms can vary depending on individual tolerance and existing gut health.
– Increased risk for certain health conditions: Individuals with specific health conditions, such as kidney disease or certain gastrointestinal disorders, may be more susceptible to the negative effects of oxalate consumption. High levels of oxalates can exacerbate symptoms and potentially worsen the condition.
4: What is the importance of oxalate ions?
Answer: Oxalate ions have several important roles and functions:
– Calcium regulation: Oxalate ions play a crucial role in calcium metabolism. They can bind with calcium ions to form calcium oxalate, which is important for the formation and
– Plant defense: In plants, oxalate ions serve as a defense mechanism against herbivores and pathogens. They can accumulate in plant tissues, particularly in leaves, as a deterrent against grazing animals and microbial attacks.
– Oxalate metabolism: Oxalate ions are involved in the metabolic process of oxalate metabolism in the human body. Oxalate is produced as a byproduct of certain metabolic pathways and is normally excreted through urine. Proper regulation and elimination of oxalate are important for maintaining normal physiological functions.
– Industrial applications: Oxalate ions are used in various industrial applications. For example, oxalic acid, derived from oxalate ions, is used as a cleaning agent, rust remover, and for some chemical processes. The ability of oxalates to bind with metals is utilized in metal cleaning and polishing.
– Analytical chemistry: Oxalate ions are used in analytical chemistry for the determination of certain metals, such as calcium and iron. They can form colored complexes with these metals, enabling their detection and quantification in various samples.
5: What is oxalate?
Answer: Oxalate is an anion (C2O42-) consisting of two carbon atoms bonded to each other through a double bond, with each carbon atom also bonded to two oxygen atoms. It is derived from oxalic acid and is commonly found in various compounds and substances.
6: Is C2O4 an oxalate?
Answer: Yes, C2O4 is the oxalate ion (also known as dioxalate or oxalate(2-)). It is a polyatomic ion composed of two carbon atoms (C) and four oxygen atoms (O) arranged in a specific structure.
7: What is the Valency of oxalate?
Answer: The valency of the oxalate ion (C2O4) is 2-. This means that it has a charge of -2 and can combine with cations, which have a positive charge, to form various oxalate compounds.
8: Is C2O4 and CO2 same?
Answer: C2O4 and CO2 are not the same. CO2 refers to carbon dioxide, which is a molecule consisting of one carbon atom (C) bonded to two oxygen atoms (O) through double bonds. It is a gas at standard temperature and pressure. On the other hand, C2O4 represents the oxalate ion, which is a polyatomic ion with a different chemical structure and valency.
9: Is C2O4 a weak acid?
Answer: C2O4 is not considered a weak acid. It is an anion and does not readily donate protons (H+ ions) in aqueous solutions to exhibit acidic properties. However, oxalic acid (H2C2O4), which can dissociate to produce the oxalate ion, is a weak acid.




