Table of Contents
S Chand Science Class 8 Solutions Chapter 14 Chemical Effects of Electric Current
Lakhmir Singh Science Class 8 Chapter 14 Very Short Answer Type Questions
Question 1.
Do liquids also conduct electricity?
Answer:
Liquids do conduct electricity, when they have free ions available like in case of Acids, Bases and Salts being dissolved in water.
Question 2.
Name two liquids which conduct electricity and two liquids which do not conduct electricity.
Answer:
Tap water and lemon water conduct electricity, this happens because tap water. while distilled water and wine do not conduct electricity.
Question 3.
Name a device which glows even when a weak electric current passes through it.
Answer:
A Light Emitting Diode (LED) glows even when a weak electric current passes through it.
Question 4.
Write the full form of LED.
Answer:
Light emitting Diode (LED)
Question 5.
How would you classify lemon juice- a good conductor or a poor conductor of electricity?
Answer:
Lemon juice is a good conductor of electricity. Lemon is a citrus fruit containing citric acid. Acids give H+ ions when dissolved in water, these free cations and anions make lemon juice a good conductor.
Question 6.
Vinegar is a sour liquid. State whether vinegar will conduct electricity or not.
Answer:
Vinegar is a poor conductor of electricity because it does not have any free ions to carry electric charge and conduct electricity.
Question 7.
What effect does an electric current produce when flowing through a conducting liquid (or conducting solution)?
Answer:
When electric current passes through a conducting liquid, it gets decomposed into ions. The conducting solution has free ions available which conduct electricity.
Question 8.
When electric current is passed through acidified water, then hydrogen and oxygen are formed. What type of effect of current is illustrated by this statement?
Answer:
When electric current is passed through acidified water, it dissociates water into H+ and OH– ions. This process is called chemical effect.
Question 9.
Acidified water is electrolyzed by using carbon electrodes. What is produced at:
(a) positive carbon electrode?
(b) negative carbon electrode?
Answer:
(a) Oxygen Gas is produced at positive carbon electrode.
(b) Hydrogen gas is produced as negative carbon electrode.
Question 10.
Give one example of the chemical effect of electric current.
Answer:
When an electric current is passed through a conducting solution, then chemical reactions take place in the solution. These chemical reactions cause chemical effects in the solution.
Example: When an electric current is passed through water, then water dissociates into hydrogen and oxygen. The chemical reaction is shown below: H2O → H+ (aq) + OH– (aq)
Question 11.
What should be done to decompose water into hydrogen and oxygen?
Answer:
Electric current must be passed through water to decompose water into hydrogen and oxygen.
Question 12.
Name the process in which a coating of one metal can be deposited on the surface of another metal by using current from a battery.
Answer:
Electroplating is the process in which a coating of one metal can be deposited on the surface of another metal by using current from a battery. The metal that needs to be deposited is used as the cathode while the metal on which it is deposited is used as anode. We use the salt of the cathode as the electrolyte.
Question 13.
Name the metal which is usually electroplated on car parts such as bumpers and bicycle handlebars made of steel.
Answer:
Chromium is electroplated on car parts such as bumpers and bicycle handlebars made of steel.
Question 14.
Which metal is electroplated on iron for making ‘cans’ used for storing food?
Answer:
Tin is electroplated on iron for making ‘cans’ used for storing food.
Question 15.
Name two metal objects which have a coating of another metal.
Answer:
- Chromium is electroplated on other metals.
- Gold is electroplated on other cheap metals.
Question 16.
Name the most common application of the chemical effect of electric current.
Answer:
The two most common examples of the chemical effect of electric current are:
- Gold Coating on cheap metals
- Electroplating of chromium metal to avoid corrosion.
Question 17.
Name two metals which are usually electroplated on cheaper metals for making jewelry (or ornaments).
Answer:
Gold and silver are electroplated on cheaper metals for making jewelry or ornaments.
Question 18.
Which is the polluting waste generated by electroplating factories?
Answer:
The conducting solution used in the electroplating process is the waste generated by electroplating factories.
Question 19.
Give a list of five objects around you which are electroplated.
Answer:
The list of five objects around you which are electroplated is:
- Kitchen utensils.
- Jewelry
- Car parts
- Cans
- Taps
Question 20.
Name two metals which are purified by using the chemical effect of current (or electrolysis).
Answer:
Copper and Gold are purified by using the chemical effect of current (or electrolysis).
Question 21.
Name two metals which are produced (or extracted) by using the chemical effect of electric current.
Answer:
Carbon and hydrogen are extracted by using the chemical effect of electric current.
Question 22.
Name one chemical compound which is produced by using the chemical effect of electric current.
Answer:
Copper sulphate is one chemical compound which is produced by using the chemical effect of electric current.
Question 23.
Name one compound which is decomposed into hydrogen and oxygen by using the chemical effect of electric current.
Answer:
Water is one compound which is decomposed into hydrogen and oxygen by using the chemical effect of electric current.
Question 24.
State whether the following statements are true or false :
(a) Rainwater is a non-conductor of electricity.
(b) A piece of fresh potato does not conduct electricity at all.
Answer:
(a) False. Rainwater is a conductor of electricity because it has dissolved ions that conduct electricity.
(b) False. A piece of fresh potato conduct electricity at all because it does not have ions to conduct electricity.
Question 25.
Fill in the following blanks with suitable words:
(a) Most liquids that conduct electricity are solutions of ……….., ……… and …………
(b) LED glows even when a …………..electric current passes through it.
(c) The passage of an electric current through a conducting solution causes………….effect.
(d) When electric current is passed through acidified water, then ……..and………..are formed.
(e) The process of depositing a layer of any desired metal on another metal by means of electricity is called ……….
(f) If you pass current through copper sulphate solution, copper gets deposited on the plate connected to the …………terminal of the battery.
Answer:
(a) Most liquids that conduct electricity are solutions of acids, base and salts.
(b) LED glows even when a low electric current passes through it.
(c) The passage of an electric current through a conducting solution causes chemical effect.
(d) When electric current is passed through acidified water, then hydrogen and oxygen are formed.
(e) The process of depositing a layer of any desired metal on another metal by means of electricity is called electroplating.
(f) If you pass current through copper sulphate solution, copper gets deposited on the plate connected to the negative terminal of the battery.
Lakhmir Singh Science Class 8 Chapter 14 Short Answer Type Questions
Question 26.
Which of the following liquids conduct electricity and which do not conduct electricity?
Lemon juice, Milk, Vinegar, Common salt solution, Sulphuric acid solution, Sugar solution, Distilled water, Honey, Sea water, Rainwater.
Answer:
Lemon juice, Milk, Vinegar, common salt solution, Sulphuric acid solution, Sea water, and Rainwater conduct electricity.
Sugar solution, Distilled water and Honey do not conduct electricity.
Question 27.
Why is it dangerous to touch a working electrical appliance with wet hands?
Answer:
Because wet hands have water and water is a good conductor of electricity. A person can get electric shock if he touches any electrical appliances with wet hands.
Question 28.
What is the advantage of using LED in testing the electrical conductivity of liquids ?
Answer:
The advantage of using LEDs is because, they glow up even if very small amount of current passes through them.
Question 29.
Which effect of electric current is utilized for detecting the flow of current through a solution:
(a) when a torch bulb is used?
(b) when a compass is used?
Answer:
(a) Heating effect of current is used detecting the flow of current through a solution.
(b) Magnetic effect of current is used detecting the flow of current through a solution.
Question 30.
What happens to the needle of a compass kept nearby when electric current is switched on in a wire? Why does this happen?
Answer:
The current carrying wire behaves like a magnet and it will cause deflection of the needle of the compass. This is called magnetic effect of electric current.
Question 31.
Explain why, distilled water does not conduct electricity but tap water conducts some electricity.
Answer:
Distilled water does not conduct electricity because it does not contain any ions which make it conducting. But it can conduct electricity when a pinch of common salt is added to it, as salt solution is conducting in nature.
Question 32.
Distilled water does not conduct electricity. What substances can be added to distilled .water in small amounts to make it a good conductor of electricity? Why?
Answer:
Lemon solution, salt, vinegar, sulphuric acid etc. can be added to added to distilled .water in small amounts to make it a good conductor of electricity. Because distilled water does not contain any ions which make it conducting. But adding these will provide free ions for conducting solution.
Question 33.
Which of the two is a better conductor of electricity: drinking water (tap water) or sea water? Give reason for your answer.
Answer:
Sea water is a better conductor because it contains sodium and chloride ions in large amounts. These ions make the solution conducting and thus, sea water is considered better conductor than the tap water which does not contain ions in large amounts.
Question 34.
Why does a brand new bicycle have shining handlebar and wheel rims? What will happen if these are accidentaly scratched?
Answer:
The shining coating is done on the metal by electroplating. When these are accidentaly scratched then, the metal will be rusted due to presence of air. So, by some metal these are coated so as to avoid rusting of iron mainly used to build the handlebars and wheel rims.
Question 35.
Is it safe for the electrician to carry out electrical repairs outdoors during heavy downpour? Explain.
Answer:
No, it is very risky and unsafe for the electrician to carry out electrical repairs outdoors during heavy downpour because rain water is conducting in nature as it contains dissolved salts. The electrician may get electrical shocks while working outdoors during rain as water may conduct electricity.
Question 36.
When the free ends of a conductivity tester (made by using a battery connected to a wire wound around a compass) are dipped into a solution, the magnetic needle shows deflection. Can you give the reason for this deflection.
Answer:
Because the free ends of a conductivity tester are dipped into a conducting solution then, it behaves like a current carrying wire and a current carrying wire behaves as a magnet because it produces magnetic field around it. And, due to this there is deflection in the compass.
Question 37.
A beaker contains an acidified copper sulphate solution. A copper plate and a carbon rod are kept in this copper sulphate solution. The copper plate is connected to the positive terminal of a battery whereas the carbon rod is connected to the negative terminal of the battery. What will you observe when an electric current is passed through this set-up for a considerable time?
Answer:
The carbon is a non-metal which is more reactive than copper. When, an electric current is passed through this set-up for a considerable time, then carbon replaces copper ions from solutions and they deposit on the negative electrode. Thus, a red-brown layer of copper metal will be deposited on the carbon rod connected to the negative terminal of the battery.
Question 38.
Does pure water conduct electricity? If not, what can we do to make it conducting?
Answer:
No. Pure water does not conduct electricity. Pure water does not contain any ions which make it conducting. But it can conduct electricity when a pinch of common salt is added to it, as salt solution is conducting in nature.
Question 39.
In case of a fire, before the firemen use the water hoses to throw water to douse fire, they shut off the electricity supply for the area. Explain why this is done.
Answer:
In case of a fire, before the firemen use the water hoses, they shut off the main electrical supply for the area because water may conduct electricity. If the electrical supply for the area is not shut off and water is poured over electrical appliances, then electricity may pass through water and harm the firemen.
Question 40 A.
Which effect of electric current is utilized when a thin layer of chromium metal is deposited on an iron tap? What is this process known as?
Answer:
This is called chemical effect of heating and the process is called as electroplating.
Question 40 B.
For electroplating copper on an iron object, which terminal of the battery (positive or negative) is connected to the iron object? Also name the electrolyte you will use for this purpose.
Answer:
In the process of electroplating, the metal to be electroplated is taken as cathode and the one used for coating is chosen as the anode. And, we know, anode is positive terminal and the cathode is negative terminal. Thus, negative terminal will be connected to the iron object.
Lakhmir Singh Science Class 8 Chapter 14 Long Answer Type Questions
Question 41 A.
What is meant by the chemical effect of electric current? Explain with the help of an example.
Answer:
When electric current passes through any conducting solution, then chemical reactions take place inside the conducting solution. This effect is called as chemical effect of electric current. Example, electroplating. The process of depositing a layer of any desired metal on another material by means of electricity is called electroplating.
Question 41 B.
Name any two applications of the chemical effect of electric current.
Answer:
- Chromium plating is done on metals body parts of cars, bikes, cycles, etc.
- Gold and silver plating is done on the artificial jewelry.
Question 41 C.
What is electrolysis? Explain why, in the electrolysis of water, ‘acidified water’ is used.
Answer:
Electrolysis is the process of dissociation of water molecules into H+ and OH– ions. When electric current Is passed through it in a conducting state. Because water does not have much higher concentration of ions. Dilute sulphuric acid is added to increase the concentration of ions in the water.
Question 42 A.
Name three types of substances in which an electric current can produce a chemical effect.
Answer:
The three types of substances in which an electric current can produce a chemical effect.
- Water
- Copper sulphate
- Vinegar.
Question 42 B.
State some of the characteristics of chemical changes brought about by the chemical effect of electric current.
Answer:
The some of the characteristics of chemical changes brought about by the chemical effect of electric current:
- The solution colour changes might take place.
- The bubbles evolve through the solution.
- The chemical deposition of one metal and replacement of the other metal.
Question 42 C.
Why does an electric bulb glow when a current passes through it?
Answer:
The electric bulb glows because it completes the circuit when put in the conducting solution and the conducting solution helps to glow the bulb.
Question 43 A.
What is meant by electroplating? What is the purpose of electroplating?
Answer:
The process of coating of a layer of any metal over other metal is called electroplating. The purpose of electroplating is to provide the shiny appearance, rust free metal surface etc.
Example
1. Chromium plating is done on metals body parts of cars, bikes, cycles, etc.
2. Gold and silver plating is done on the artificial jewelry.
Question 43 B.
Which properties of chromium metal make it suitable for electroplating it on car bumpers, bath taps and bicycle handlebars, etc., made of iron?
Answer:
The chromium metal is suitable for electroplating it on car bumpers, bath taps and bicycle handlebars, etc., made of iron because it is corrosion resistant and also gives shiny appearance.
Question 44.
A strip of impure copper metal is given to you. Describe briefly how you will purify it by using the chemical effect of electric current. Draw a labelled diagram of the experimental set up used for this purpose.
Answer:
Copper ion is positively charged. It will be attracted towards the plate which is connected to the negative terminal of the battery. Because positive charges attract negative charges and repel positive charges.
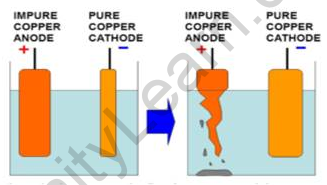
As copper ions from the impure copper electrode are transferred to the thin copper plate, this thin pure copper plate must be connected to the negative terminal of the battery. Consequently, impure copper rod is connected to the positive terminal of the battery. In this way, pure copper is deposited on the negative electrode i.e. cathode.
Question 45.
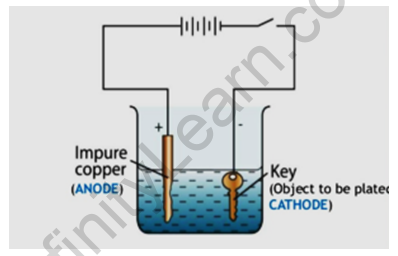
With the help of a labelled diagram, describe briefly how an iron key can be electroplated with copper.
Answer:
We need two electrodes made from different conducting materials (here copper and iron as anode and cathode respectively), an electrolyte, and electricity supply. The iron key will be taken as the cathode (negative electrode ) and the copper to be electroplated on the iron key to be made the anode (positive electrode). When we open the electricity, then copper ions are deposited on the negative electrode and in this way copper is electroplated on the iron key.
Lakhmir Singh Science Class 8 Chapter 14 Multiple Choice Questions (MCQs)
Question 46.
In an activity to check the conduction of electricity through two liquids labelled A and B by using a bulb, it is observed that the bulb glows brightly for liquid A while it glows very dimly for liquid B.
A. Liquid A is a better conductor than liquid B
B. Liquid B is a better conductor than liquid A
C. Both liquids are equally conducting
D. Conducting properties of liquids cannot be compared in this manner
Answer:
A. Liquid A is a better conductor than liquid B
Because current flows more easily throughout liquid A than B. Thus, A is a better conductor than liquid B.
Question 47.
Which of the following does not conduct electricity?
A. Vinegar solution
B. Sugar solution
C. Lemon juice solution
D. Caustic soda solution
Answer:
B. Sugar solution
Sugar solution does not contain any ions. Thus, it does not conduct electricity.
Question 48.
Which of the following metals should be electroplated on a tiffin box made of steel?
A. copper
B. chromium
C. silver
D. tin
Answer:
D. tin
Tin should be electroplated on a tiffin box made of steel as it is non-corrosive.
Question 49.
Which of the following metals are produced by the electrolysis of their molten compounds?
A) Copper
B) Silver
C) Aluminium
D) Sodium
A. A and B
B. Band C
C. C and D
D. only D
Answer:
C. C and D
Both Aluminum and Sodium metals are produced by the electrolysis of their molten compounds because they are not freely available. They need to be extracted from their sources.
Question 50.
Which of the following types of energy can be used to decompose water into its elements?
A. heat energy
B. light energy
C. chemical energy
D. electrical energy
Answer:
D. electrical energy
Electrical energy is used to decompose water into its elements i.e. hydroxide (OH–) and (H+) ions.
Question 51.
Which of the following compounds is manufactured by using the chemical effect of electric current?
A. ammonium hydroxide
B. sodium carbonate
C. magnesium hydroxide
D. sodium hydroxide
Answer:
D. sodium hydroxide
Sodium hydroxide (NaOH) is manufactured by using the chemical effect of electric current when a sodium salt is electrolysed.
Question 52.
Which of the following objects should not be chrome-plated ?
A. car bumper
B. gas stove
C. frying pan
D. bicycle bell
Answer:
C. frying pan
Frying pan should not be chrome-plated because it can enter the body by mixing in the food which can prove to be dangerous for the body.
Question 53.
Which of the following is a weak electrolyte?
A. carbonic acid
B. sodium hydroxide
C. copper sulphate
D. nitric acid
Answer:
A. carbonic acid
Carbonic acid is a weak electrolyte because it does not completely dissociate into the aqueous solution.
Question 54.
Electrolytes conduct electricity due to the movement of:
A. electrodes
B. atoms
C. electrons
D. ions
Answer:
D. ions
Electrolytes conduct electricity due to the movement of ions. Because positive and negative ions conduct electricity.
Question 55.
Non-metals are generally non-conductors of electricity. The non-metal whose one of the forms can be used to make electrodes in electrolysis experiments is:
A. iodine
B. carbon
C. silicon
D. phosphorus
Answer:
B. carbon
The non-metal whose one of the forms can be used to make electrodes in electrolysis experiments is Carbon and the form is graphite form. We come across graphite electrodes because it is a good conductor.
Question 56.
The decomposition produced by passing current through a conducting liquid is called:
A. dialysis
B. hydrolysis
C. electrolysis
D. electroplating
Answer:
C. electrolysis
Electrolysis is the decomposition produced by passing current through a conducting liquid. The solution is dissociated into ions.
Question 57.
Which of the following is not an application of the chemical effect of electric current?
A. electroplating of metals
B. purification of metals
C. decomposition of elements
D. decomposition of compounds
Answer:
C. decomposition of elements
Decomposition of elements is not an application of the chemical effect of electric current. Electroplating, purification and decomposition of compounds can be done with the help of chemical effect of electric current.
Question 58.
One of the following is not used for electroplating metal articles. This one is :
A. nickel
B. chromium
C. sodium
D. silver
Answer:
C. sodium
Sodium is not used for electroplating metal articles. Because it is a corrosive metal.
Question 59.
An arrangement having two carbon rods kept in a conducting liquid in a vessel is known as:
A. rechargeable cell
B. storage cell
C. biological cell
D. electrolytic cell
Answer:
D. electrolytic cell
An electrolytic cell is an arrangement having two carbon rods kept in a conducting liquid in a vessel. This is the process setup for the electrolysis of electrolytes.
Question 60.
Which of the following effects is not produced by the chemical reactions brought about by an electric current?
A. bubbles of gases on electrodes
B. deposits of metals on electrodes
C. change in color of solution
D. formation of a precipitate
Answer:
D. formation of a precipitate
Formation of a precipitate is not produced by the chemical reactions brought about by an electric current. Because it does not involves any chemical reaction but involves chemical changes in the solutions
Question 61.
Which of the following can be electroplated with chromium?
A) Bakelite
B) Graphite
C) Steel
D) Teflon
A. A and B
B. B and C
C. only C
D. B and D
Answer:
B. B and C
Graphite and Steel can be electroplated with chromium. Chrome plating is not done on graphite and steel electrodes.
Question 62.
In order to obtain a coating of silver metal on a flower vase made of copper, the electrolyte has to be:
A. silver nitrate solution
B. copper nitrate solution
C. sodium nitrate solution
D. copper sulphate solution
Answer:
A. silver nitrate solution
In order to obtain a coating of silver metal on a flower vase made of copper, the electrolyte has to be silver nitrate solution. Because the silver ions will come from the electrolyte.
Question 63.
The process of purification of an impure metal is like:
A. electroplating a metal on the same type of metal
B. electroplating a metal on another type of metal
C. producing a chemical compound by electrolysis
D. decomposing a chemical compound by electrolysis.
Answer:
A. electroplating a metal on the same type of metal
The process of purification of an impure metal is like electroplating a metal on the same type of metal. The pure metal is removed and deposited on the pure metal.
Question 64.
The device which can be used to detect very small current flowing in an electric circuit is:
A. LEAD
B. dB
C. MCB
D. LED
Answer:
D. LED
LED Light emitting diode is used to detect very small current flowing in an electric circuit.
Question 65.
If plus sign (+) denotes the positive electrode and minus sign (-) denotes the negative electrode, then which of the following statement is correct for an iron spoon to be copper-plated ?
A. Iron spoon (+),copper plate(-), Iron sulphate electrolyte
B. Iron spoon(-), copper plate(+), Iron sulphate electrolyte
C. Copper plate(-), Iron spoon(+), Copper sulphate electrolyte
D. Copper plate(+), Iron spoon(-), Copper sulphate electrolyte
Answer:
D. Copper plate(+), Iron spoon(-), Copper sulphate electrolyte
Copper plate(+), Iron spoon(-), Copper sulphate electrolyte is the correct setup combination for electroplating. Because the anode is made the metal to be deposited and cathode to the one on which it is electroplated.
Lakhmir Singh Science Class 8 Chapter 14 Questions Based On High Order Thinking Skills (HOTS)
Question 66.
A student staying in a coastal region tests the drinking water and also the sea water ‘With a circuit in which a part of the connecting wire is wound around a matchbox containing compass. He finds that the compass needle deflects more in the case of sea water. Can you explain the reason?
Answer:
Sea water is a better conductor because it contains sodium and chloride ions in large amounts. These ions make the solution conducting and thus, sea water is considered better conductor than the drinking water which does not contain ions. The ions of drinking water are removed while removed while purifying it.
Question 67.
When an electric current is passed through a cut potato for a considerable time, a coloured spot is formed around one of the electrodes :
(a) What is the colour of the spot?
(b) Around which electrode (positive or negative electrode) the coloured spot is formed?
(c) Which effect of electric current is involved in this case?
Answer:
(a) The spot color is Greenish blue.
(b) The coloured spot is formed around the positive electrode.
(c) This is known as Chemical effect.
Question 68.
In the process of purification of copper metal, a thin plate of pure copper and a thick rod of impure copper are used as electrodes, and a metal salt solution is used as an electrolyte:
(a) Which electrode is connected to the positive terminal of the battery?
(b) Which electrode is connected to the negative terminal of the battery?
(c) Which metal salt solution is taken as electrolyte?
Answer:
(a) Thick rod of impure copper is connected to the positive terminal of the battery.
(b) Thin plate of pure copper is connected to the negative terminal of the battery.
(c) Copper sulphate solution is taken as electrolyte.
Question 69.
A student had heard that rainwater is as good as distilled water. So, he collected some rainwater in a clean glass beaker and tested it. To his surprise he found that the compass needle showed deflection. What could be the reason?
Answer:
Rain water can collect salts from the atmosphere has some dissolved salts whereas the distilled water does not. Thus, the rainwater showed the deflection because it conducts electricity.
Question 70.
Name three liquids which when tested in the manner shown in figure given along side may cause the magnetic needle of compass to deflect.
Answer:
The Liquids that conduct electricity will cause the magnetic needle to deflect because the electric current is capable of producing magnetic effect. The liquids like lemon juice, salt water and vegetable oil allow electricity to pass through them. Hence, these liquids can be used as in the beaker to show the given effect.









