Table of Contents
Introduction to Acetyl Chloride
Acetyl chloride (CH3COCl) is an acyl chloride produced from acetic acid (CH3COOH). It belongs to the class of organic chemicals known as acid halides. It is a colourless, corrosive, and volatile liquid. Its formula is usually shortened to AcCl. Acetyl chloride, also known as ethanoyl chloride, is an important organic compound with various industrial applications.
In this article, we will explore the structure, uses, preparation methods, and reactions of acetyl chloride in detail.
Acetyl Chloride Formula and Structure
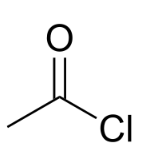
The acetyl chloride formula is C2H3ClO, and the acetyl chloride structure consists of a carbonyl group (C=O) bonded to a chlorine atom (Cl). The carbonyl carbon is also attached to a methyl group (CH3).
Acetyl Chloride IUPAC Name
The acetyl chloride IUPAC name is ethanoyl chloride
Preparation of Acetyl Chlorid
- Acetic acid to acetyl chloride
Acetyl chlorides are most commonly synthesized by reacting acetic acid with thionyl chloride. In this process, dimethylformamide is utilized as a catalyst.
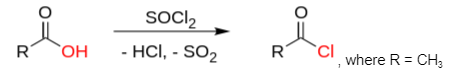 , where R = CH3
, where R = CH3
- Acetic anhydride to acetyl chloride
In the industrial process, acetic anhydride undergoes a reaction with hydrogen chloride to yield acetyl chloride and acetic acid.
The chemical equation for this reaction is:
(CH3CO)2O + HCl → CH3COCl + CH3CO2H
Physical Properties of Acetyl Chloride
- Acetyl Chloride CAS No.: The CAS number of acetyl chloride is 75-36-5.
- Acetyl Chloride Density: The density of acetyl chloride is approximately 1.10 g/cm³.
- Acetyl Chloride Molecular Weight: The molecular weight of acetyl chloride is approximately 78.5 g/mol.
- Acetyl chloride Solubility: It is soluble in chemical solvents like alcohol, ether, and chloroform.
- Melting and boiling values: Acetyl chlorides have lower boiling and melting points than their corresponding acids due to their inability to form hydrogen bonds.
Chemical Properties and Reactions of Acetyl Chloride
Acetyl chloride participates in numerous reactions, including acylation, nucleophilic substitution, and hydrolysis. These reactions make it a valuable reagent in organic chemistry.
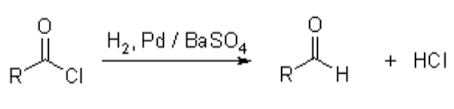
- Acetyl Chloride to Acetaldehyde
Acetyl chloride is reduced by hydrogen with BaSO4 to an aldehyde in the presence of palladium. This is called the Rosenmund Reaction.
- Preparation of Acetic Acid from Acetyl Chloride
The conversion from acetyl chloride to acetic acid happens through hydrolysis. When acetyl chloride is treated with water, it undergoes a reaction to form acetic acid and hydrogen chloride gas. Acetic acid is an essential compound in various chemical processes and the main component of vinegar.
The chemical equation for this reaction is:
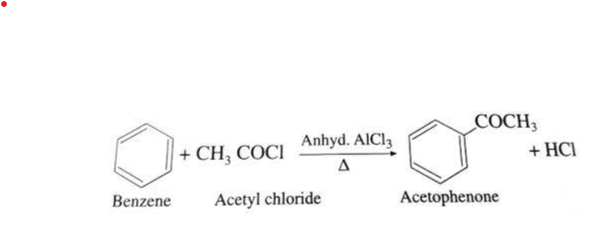
CH3COCl + H2O → CH3COOH + HCl - Benzene Reacts with Acetyl Chloride
Benzene, a widely used aromatic hydrocarbon, can react with acetyl chloride in the presence of an aluminum chloride catalyst to form acetylbenzene, also known as acetophenone. The chemical reaction is as follows:
- Acetyl Chloride to Acetone
An excellent yield of acetone is achieved when an acetyl chloride is treated with a diorgano cuprate (Gillman) reagent ((CH3)2CuLi).
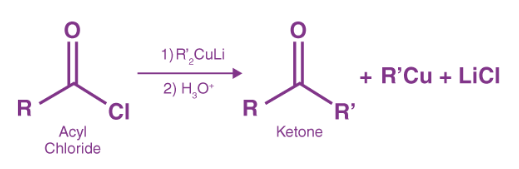
Where R and R’ represent CH3.
Uses of Acetyl Chloride
- Acetyl chloride finds application in the synthesis of various organic compounds, such as acetic anhydride and acetamide.
- It is also used in the pharmaceutical industry and in the production of dyes, perfumes, and agrochemicals.
- Acetyl chloride is frequently used in liquid formulations for optical displays and memory devices.
Frequently Asked Questions on Acetyl Chloride
What is the purpose of acetyl chloride?
Acetyl chloride is a reagent used in the derivatization of alcohols and amines to prepare acetic acid esters and amides.
What is the most effective way to make acetyl chloride?
Acetic acid is heated with phosphorus trichloride, phosphorus pentachloride, or thionyl chloride to produce acetyl chloride. Thionyl chloride is a better reagent since its byproducts are gaseous and easily separated.
What causes acetyl chloride to be more reactive?
Acetyl chlorides are highly reactive because the carbon atom is relatively positive and attracts nucleophiles while being connected to both highly electronegative oxygen and chlorine atoms. They have several reactions, but they are all basically the same.
Is it true that acetyl chlorides are acidic?
Anhydrous acetyl chloride is neutral because there is no H+ to give. However, in an aqueous medium, acetyl chloride can react with water to form carboxylic acid and HCl.
Are acetyl chlorides dangerous?
Acetyl chlorides are generally hazardous and must be handled with extreme caution. They can react with the water on the surface of the eye, producing hydrochloric and organic acids that irritate the eye.









