Table of Contents
Coulomb’s law, also known as Coulomb’s inverse-square law, is a physical experiment that quantifies the amount of force between two stationary, electrically charged particles. The electric force between two charged bodies at rest is commonly referred to as electrostatic force or Coulomb force. Although the law existed prior to 1785, it was published for the first time by French physicist Charles-Augustin de Coulomb, hence the name. Coulomb’s law was critical to the development of electromagnetism theory, possibly even its starting point, because it allowed for meaningful discussion of the quantity of electric charge. The electrostatic force of attraction or repulsion between two point charges is directly proportional to the product of their magnitudes and inversely proportional to the square of their distance, according to the law.
K or ke is Coulomb’s constant (ke ≈ 8.988×109 N⋅m2⋅C−2), q1 and q2 are the signed magnitudes of the charges, and r is the distance between the charges.
The force is directed in the direction of the straight line connecting the two charges. The electrostatic force between two charges is repulsive if their signs are the same; if their signs are opposite, the force is attractive. . The law is analogous to Isaac Newton’s inverse-square law of universal gravitation because it is an inverse-square law, but gravitational forces are always attractive, whereas electrostatic forces can be either attractive or repulsive. Coulomb’s law can be used to calculate Gauss’ law and vice versa. The two laws are equivalent in the case of a single stationary point charge, expressing the same physical law in different ways. The law has been thoroughly tested, and observations on a scale ranging from 10−16 m to 108 m have confirmed its validity.
Join Our Online Courses for JEE: JEE Class 12 Online Course | JEE Dropper Online Course
Overview
According to Coulomb’s law, the force of attraction or repulsion between two charged bodies is directly proportional to the product of their charges and inversely proportional to the square of their distance. It acts along the line that connects the two charges considered to be point charges. In 1785, a French physicist named Charles Augustin de Coulomb coined the term “tangible relationship” to refer to a mathematical relationship between two electrically charged bodies. Coulomb’s law, also known as Coulomb’s inverse-square law, is an equation for the force that causes bodies to attract or repel each other. A coulomb is defined as the charge that repels an equal charge of the same sign with a force of 9×109 N when the charges are separated by one metre in a vacuum. Coulomb force is a mutual and internal force that is conservative. The value of εo is 8.86 × 10-12 C2/Nm2 (or) 8.86 × 10-12 Fm–1
Both gravitational and electric forces decrease with the square of the distance between the objects, and both act along a line that connects them. Coulomb’s law, on the other hand, determines the magnitude and sign of an object’s electric force based on its electric charge rather than its mass. As a result, charge determines how electromagnetism affects the motion of charged objects. A charge is a fundamental property of matter. Every constituent of matter carries an electric charge that can be positive, negative, or zero. For example, electrons are negatively charged, whereas atomic nuclei are positively charged. The bulk matter has an equal amount of positive and negative charges, resulting in a net charge of zero.

JEE Foundation Class for 10
JEE Foundation Class for 10 enhances critical thinking and problem-solving skills through engaging activities and advanced learning techniques, ensuring academic excellence.
Coulomb defined the electric force as having the following properties for charges at rest: Charges with similar charges repel each other, while charges with dissimilar charges attract each other. As a result, two negative charges repel each other, whereas a positive charge attracts a negative charge.
The attraction or repulsion is directed along the line formed by the two charges. The magnitude of the force is proportional to the square of the distance between the two charges. As a result, increasing the distance between the two charges by a factor of two reduces the attraction or repulsion to one-fourth of its original value. When the charges are 10 times closer together, the force multiplies by a factor of 100.
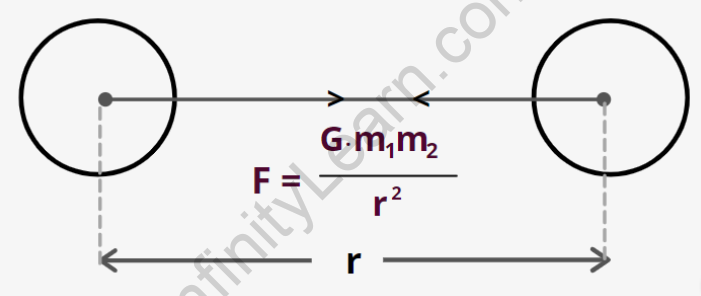
Coulomb’s law forces between two point charges
Natural gravitational force and electric force are concepts. The strengths can be easily compared by determining the ratio of forces in different cases.Coulomb’s law states that the force of attraction or repulsion between two charged things is directly proportional to the product of their charges and inversely proportional to the square of their distance.
It has an effect on the line that connects the two charges which are called point charges.
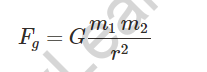
Where m1 and m2 are the masses of two bodies separated by r.
The gravitational constant is symbolised by the letter G.
Electrostatic force
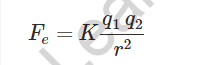
Gravitational Force – According to the universal law of gravitation, every object in the universe attracts another object with a force equal to its mass.
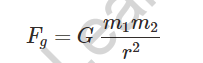
It obeys the inverse square law, which states that gravitational force is always attractive in nature.
Coulomb’s law states that two charged particles with charges q1 and q2 separated by a distance r exert an electrostatic force on each other given by
![]()

k has the value 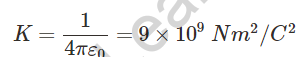
It can be either attractive or repulsive in nature; for similar charges, it is repulsive; for opposite charges, it is attractive.
Coulomb’s law states that “the force of interaction between two point charges is directly proportional to the product of their magnitudes and inversely proportional to the square of their separation distance.”

JEE Foundation Class for 9
JEE Foundation Class for 9 enhances critical thinking and problem-solving skills through engaging activities and advanced learning techniques, ensuring academic excellence.
Limitations of Coulomb’s Law
- The law only applies to point charges at rest.
- Coulomb’s Law can only be applied when the inverse square law is followed.
- When charges have an arbitrary shape, it is difficult to apply Coulomb’s law because we cannot determine the distance’ between the charges.
- The law cannot be directly applied to calculating the charge on the big planets.
FAQs
What is the force of Coulomb's law between two point charges?
The force of attraction or repulsion between two charged bodies is directly proportional to the product of their charges and inversely proportional to the square of the distance between them, according to Coulomb's law. It acts along the line that connects the two charges that are considered to be point charges.
What is charge and how does it explain the force between two charges?
Coulomb defined the electric force for charges at rest as having the following properties: Charges that are similar repel each other, while charges that are dissimilar attract each other. As a result, two negative charges repel each other, whereas a positive charge attracts a negative charge. The attraction or repulsion is directed along the line formed by the two charges.








