Table of Contents
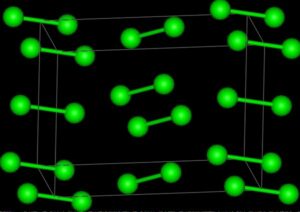
In chemistry, triiodide typically refers to the triiodide particle, I3. This anion, one of all the polyhalogen ions, consists of 3 iodine atoms. It’s fashioned by combining liquid solutions of halide salts and Iodine. Some salts of the ion are isolated, together with thallium(I) triiodide (Tl+[I3]−) and ammonia triiodide ([NH4]+[I3]−). Triiodide is ascertained to be a red colourized resolution.
Join Our Courses: JEE Class 11 Students | JEE Class 12 Students | JEE Dropper
STRUCTURE AND BONDING
The particle is linear and symmetrical. According to VSEPR theory, the central iodine atom has 3 lone equatorial pairs, and therefore the terminal iodine atoms square measure secured axially in a very linear fashion because of the 3 lone pairs bonding to the central iodine-atom. Within the molecular orbital model, a typical clarification for the hypervalent bonding on the central Iodine involves a three-centre four-electron bond. The I−I bond is longer than in matter iodine, I2.
In ionic compounds, the bond lengths and angles of triiodide vary reckoning on the character of the ion. The triiodide ion is polarised, and in several salts, one I−I bond becomes shorter than the opposite. Solely together with giant cations, e.g. quaternary ammonia like [N(CH3)4]+, could the triiodide stay roughly symmetrical?

JEE Foundation Class for 10
JEE Foundation Class for 10 enhances critical thinking and problem-solving skills through engaging activities and advanced learning techniques, ensuring academic excellence.
PROPERTIES
The triiodide particle is the simplest polyiodide; many higher polyiodides exist. In the resolution, it seems yellow at low concentrations and brown at higher concentrations. The triiodide particle is blamed for the well-known chromatic colour that arises once iodine solutions act with starch. Halide doesn’t react with starch, nor do solutions of Iodine in nonionic solvents.
Lugol’s Iodine contains iodide, and a ratio quantity of elemental Iodine, so vital amounts of triiodide particles exist during this resolution.
HYBRIDIZATION OF I3
To know the crossbreeding of Triiodide particle, we will use a straightforward crossbreeding formula that is given as;
Number of crossbreeding = electron + monovalent + (negative charge) – (positive charge)/2
If we glance at the iodine atoms, there are seven valence electrons in its outer shell, and 2 monovalent atoms are gifts. Further, throughout the mix of Iodine with the 2 alternative Iodine atoms, the central atom gains a charge whose price is taken as one.
If we tend to place or substitute the values per the formula, we get
7+1+2/2
=10/2
=5
Therefore the crossbreeding range is up to five. Currently, we will say that crossbreeding is sp3d.
Alternatively, we will conjointly confirm the crossbreeding of I3- by knowing the number of valence electrons and lone pairs and conniving their total. In this case, if we take into account the lone pairs, there are three such pairs, whereas the amount of atoms donating valence electrons is two. If we add these values, we tend to get five which might be taken as sp3d crossbreeding.
Tincture of Iodine, though nominally an answer of elemental Iodine in ethyl alcohol, conjointly contains vital amounts of triiodide because of its content of each halide and water.
FAQs
What is the hybridization of I3 plus?
The structure of I3+ is bent or formed, and the hybridization is sp3.
Is I3 one Polar?
I3- is made by the reaction of I2 (Iodine) with I- (iodide). after you add Iodine crystals to an answer of iodide in water, you'll generate a good quantity of KI3 (potassium triiodide). I3- is most positively polar since it's an anion and carries an electric charge.
How many lone pairs will I3 have?
3 lone pairs.