Table of Contents
Silver is a fascinating element that has been prized for its beauty and versatility for centuries. With its brilliant luster and valuable properties, silver has played a significant role in shaping human history and continues to be an important metal in various industries today.
In this blog post, we will explore the definition, symbol, uses, properties, atomic number, and some interesting facts about silver that make it such a remarkable element. Whether you’re a science enthusiast or simply curious about this precious metal, read on to discover more about the amazing world of silver.
Also Check: Science About Nitrogen
What is Silver – A Definition
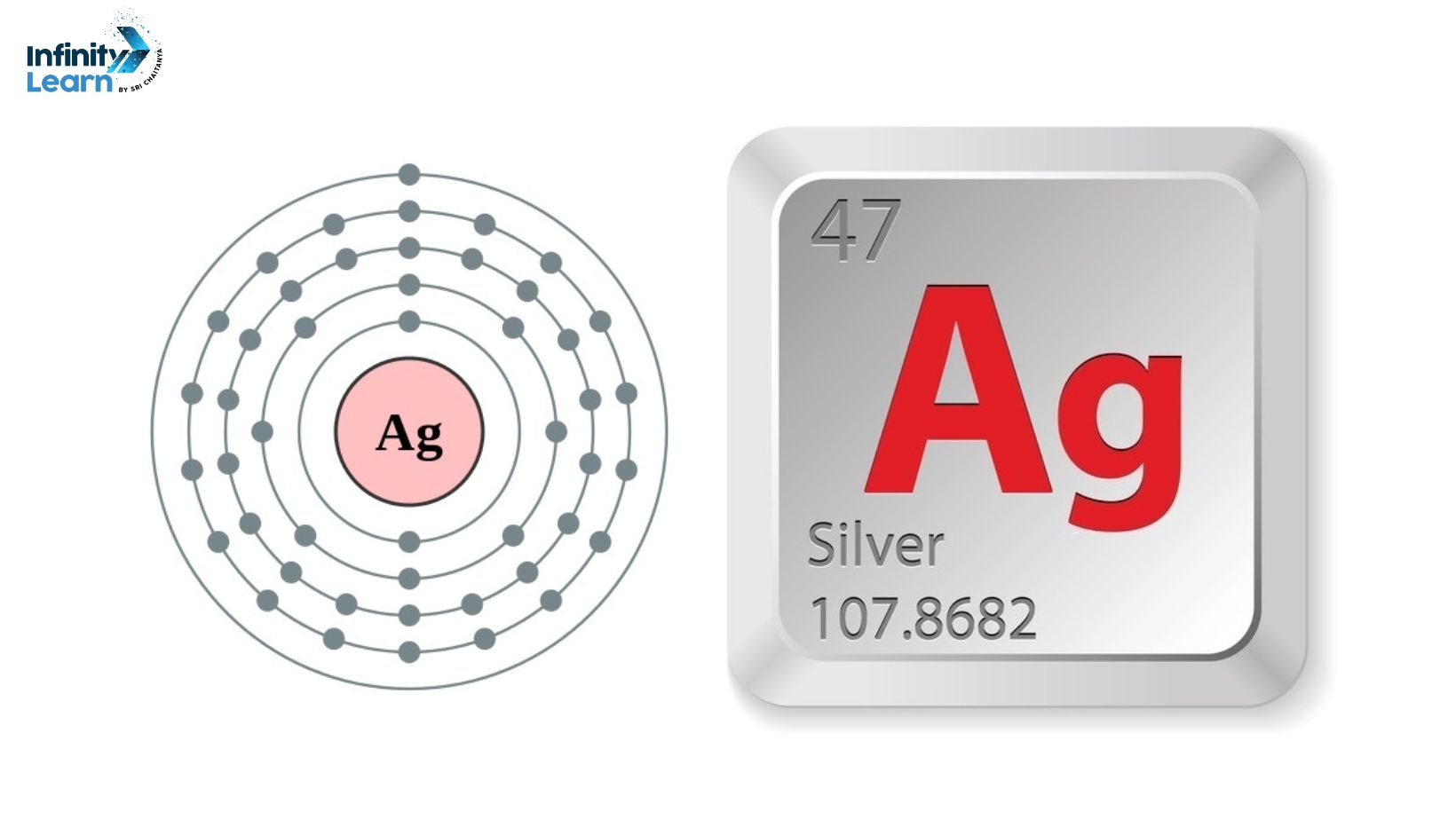
Silver is a fascinating element with a long and rich history. It is a precious metal known for its brilliant luster and remarkable properties. But what exactly is silver? In simple terms, silver is a chemical element represented by the symbol Ag on the periodic table. It belongs to the group of transition metals and has an atomic number of 47.
From a broader perspective, silver is much more than just a shiny metal. It holds great cultural and economic significance. Throughout history, silver has been used for currency, jewelry, and decorative objects. It has been valued for its beauty and rarity, often associated with wealth and status.
 silver
silver
Silver also has numerous practical applications in various industries. It is widely used in electrical components, mirrors, solar panels, and even medical devices. Its exceptional thermal and electrical conductivity, as well as its antimicrobial properties, make it highly sought after.
Also check: Mercury Element
In addition to its uses, silver possesses unique physical and chemical properties. It is a soft and malleable metal that can be easily shaped into intricate designs. It has a high reflectivity, allowing it to efficiently reflect light. Silver is also a great conductor of heat and electricity, making it an essential material in electronics.
Overall, silver is an element that has captivated humans for centuries. Its definition extends beyond its scientific characteristics, encompassing its historical, cultural, and practical significance.
The Symbol of Silver – ‘Ag’
The symbol of silver, ‘Ag’, is derived from the Latin word “argentum,” meaning silver. This symbol has been used for centuries to represent this remarkable element. But what does ‘Ag’ really mean?
The symbol ‘Ag’ is not just a random combination of letters. It represents the unique properties and significance of silver. The letter ‘A’ represents the first letter of the Latin word “argentum,” while the letter ‘g’ stands for “generation,” indicating the everlasting impact of silver across generations.
The symbol ‘Ag’ is internationally recognized and widely used in scientific and industrial contexts. It allows scientists and researchers to easily identify and refer to silver in their studies and experiments.
Also Check: Stegosaurus
In addition to its scientific significance, the symbol ‘Ag’ also carries a sense of prestige and value. It symbolizes wealth, beauty, and elegance. When you see ‘Ag’ on jewelry or decorative objects, it immediately signifies the presence of silver and its inherent desirability.
Diverse Uses of Silver
Silver is a remarkably versatile metal that finds its application in various industries. Its unique properties make it highly sought after in different fields.
One of the primary uses of silver is in jewelry. Silver jewelry has been adorning men and women for centuries, admired for its timeless beauty and elegance. From intricate necklaces to delicate rings, silver jewelry is a popular choice for those who appreciate its lustrous charm.
Another important use of silver is in the electrical industry. Silver is an excellent conductor of electricity, and it is used in electrical contacts, switches, and connectors. Its high conductivity allows for the efficient flow of electricity, making it an essential component in various electronic devices.
Silver also plays a crucial role in the medical field. Its antimicrobial properties make it ideal for use in medical equipment and devices. Silver coatings are applied to medical instruments and implants to prevent the growth of bacteria and reduce the risk of infections.
Also Check: Friction
In addition, silver has applications in photography, where it is used in the production of photographic films and papers. It is also used in the production of mirrors, as the reflective coating on the back of the glass is often made of silver.
Furthermore, silver has a significant role in the solar energy industry. It is used in the production of solar panels due to its ability to efficiently convert sunlight into electricity. Silver’s high conductivity and durability make it an ideal material for this renewable energy source.
Lastly, silver is also used in various industrial processes, including chemical production, water purification, and catalysis. Its versatility and effectiveness make it a valuable component in these applications.
Physical and Chemical Properties of Silver
Silver possesses a unique combination of physical and chemical properties that contribute to its remarkable versatility and desirability. On a physical level, silver is a soft and malleable metal that can be easily shaped into various forms. Its ductility allows it to be drawn into thin wires, making it an excellent choice for electrical conductivity. Silver is also highly reflective, with the ability to efficiently reflect light. This property makes it ideal for mirrors and other reflective surfaces.
Chemically, silver is known for its high corrosion resistance. Unlike other metals that easily tarnish, silver retains its brilliant luster over time. This makes it a popular choice for jewelry and decorative objects. Silver also has excellent thermal conductivity, allowing it to efficiently transfer heat. This property is crucial in applications such as heat sinks in electronic devices.
Also Check: Speed and Velocity
Another fascinating chemical property of silver is its antimicrobial effect. Silver ions can kill bacteria and other microorganisms, making them useful in medical applications and water purification processes.
Overall, the physical and chemical properties of silver make it an invaluable material in various industries. Its versatility, durability, and aesthetic appeal have contributed to its enduring popularity throughout history.
Atomic Number and Atomic Weight of Silver
The atomic number of silver is 47, and its atomic weight is 107.87 amu. The atomic number represents the number of protons in the nucleus of a silver atom, while the atomic weight represents the average mass of all the isotopes of silver.
Silver has two naturally occurring isotopes: silver-107 and silver-109. Silver-107 is the most abundant isotope, making up approximately 51% of the total silver in nature. Silver-109 accounts for the remaining 49%. These isotopes differ in their number of neutrons, with silver-107 having 60 neutrons and silver-109 having 62 neutrons.
The atomic weight of silver is calculated by taking into account the abundance of each isotope and its respective atomic mass. This gives us an average value that represents the overall atomic weight of silver.
Also Check: How Do Plants Grow
Understanding the atomic number and atomic weight of silver helps scientists and researchers study its properties and behavior. It also plays a crucial role in determining the chemical reactivity and bonding of silver with other elements.
Fascinating Facts About Silver
Silver is not only a beautiful and versatile metal, but it is also filled with fascinating facts that make it even more intriguing.
Here are some captivating facts about silver that will deepen your appreciation for this remarkable element:
- Silver has the highest electrical conductivity of any metal, making it an essential component in the production of electrical and electronic devices. Its conductivity is surpassed only by superconductors at extremely low temperatures.
- The word “sterling” comes from the Old Norman word “esterling,” meaning “little star.” This term was used to describe silver pennies minted in Norman England and was later applied to all high-quality silver.
- Silver is antibacterial and antimicrobial. It can kill a wide range of harmful bacteria, making it valuable in medical applications and water purification.
- Silver has been used as a form of currency for thousands of years. In ancient times, silver coins
were widely used for trade and commerce, and silver continues to be a popular investment today.
- Silver is a noble metal, meaning it is resistant to corrosion and oxidation. This property contributes to its long-lasting luster and durability.
- Silver is believed to have mystical and healing properties. Many cultures throughout history have used silver for its supposed ability to ward off evil spirits, promote good health, and bring good luck.
- Silver is the most reflective element, reflecting about 95% of visible light. This property is utilized in mirrors and reflective coatings.
- The largest silver nugget ever found weighed a whopping 2,740 pounds and was discovered in Mexico in 1983.
- Silver is an important element in photography. Silver halide crystals are used in the film, and the development process involves the reaction of silver with light to create images.
- Silver jewelry is not only beautiful but also has health benefits. Some believe that wearing silver jewelry can help alleviate certain health conditions, such as arthritis and allergies.
These fascinating facts only scratch the surface of the wonders of silver. From its historic significance to its practical applications and remarkable properties, silver continues to captivate us with its timeless allure.
Silver FAQs
Is silver a metal or non-metal?
Silver is a metal, categorized in Group 11 of the periodic table along with gold and copper.
What is the common oxidation state of silver?
The most common oxidation state of silver is +1.
Does silver tarnish?
Yes, silver tarnishes over time due to its reaction with sulfur compounds in the air, forming a layer of silver sulfide on the surface.
Is silver a good conductor of electricity?
Yes, silver is an excellent conductor of electricity, even better than copper.
Can silver be alloyed with other metals?
Yes, silver is often alloyed with metals like copper to create sterling silver, enhancing its durability and strength.
What is the significance of silver nanoparticles in nanotechnology?
Silver nanoparticles have antimicrobial properties, making them valuable in various applications, including wound dressings and water purification.
How is silver extracted from its ores?
Silver is commonly extracted from its ores through a process called cyanidation, where a cyanide solution is used to dissolve and extract silver from its ore.








