Table of Contents
Section A
1.How is dialysis carried out? Mention one application of it.
2.Name the process of heating sulphide ore in the presence of oxygen so as to convert it into oxide. Give one equation too.
3. Complete the following equation.
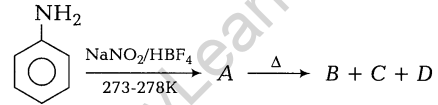
4.How can you say that D-glucose is a reducing sugar?
5.In automobile, there is ABS (Airbag System), which works on releasing a gas.
- Name the gas released.
- Provide the source
Section B
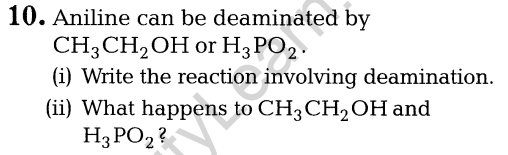
6. How will you convert ethanol to propanone?
(ii) Can Gattermann-Koch reaction be considered similar to Friedel-Crafts acylation?

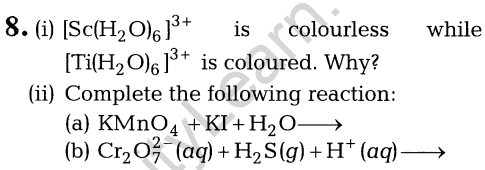

Section C
11.(i) How would you account for the following?
- Frenkel defects are not found in alkali metal halides.
- Schottky defects lower the density of related solids.
(ii) What happens when ferrimagnetic Fe3 04 is heated at 850 K and why?
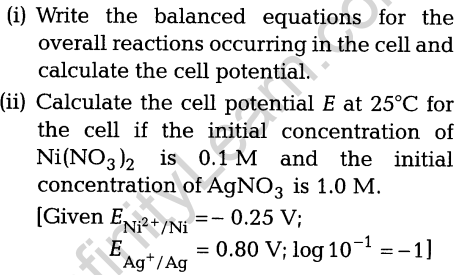
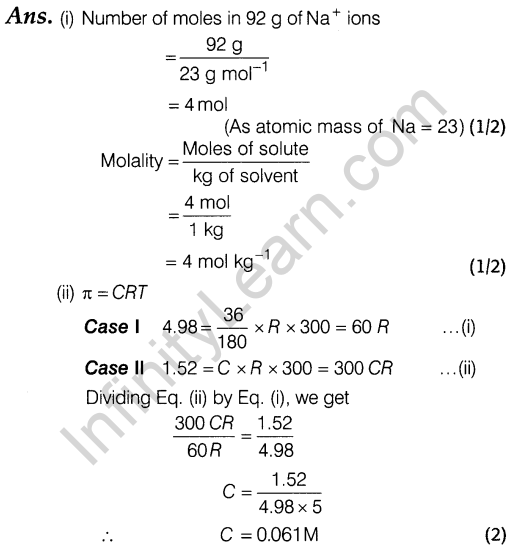
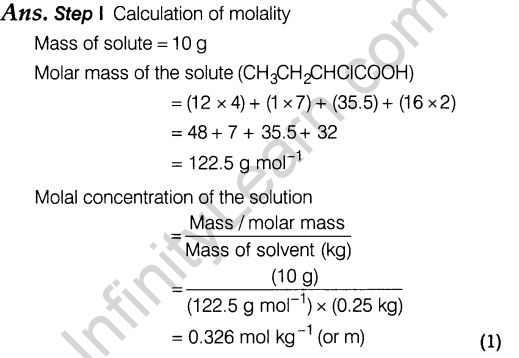
12. (i) If the density of lake water is 1.25 g mL-1 and it contains 92 g of Na+ ions per kg of water, calculate the molality of Na+ ions in the lake.
(ii) At 300 K, 36 g of glucose present per litre in its solution has an osmotic pressure of 4.98 bar. If the osmotic pressure of the solution is 1.52 bar at the same temperature, what would be its concentration?
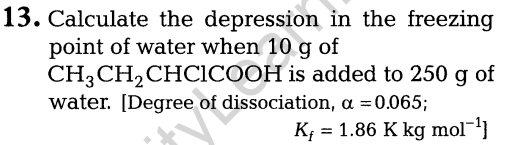
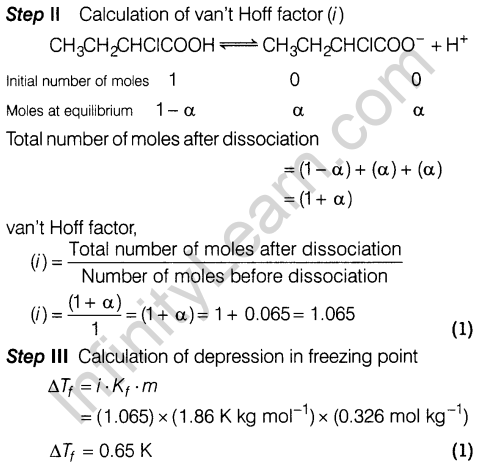
14. For the complex, [Fe(en)2Cl2]Cl (en = ethylenediamine), identify
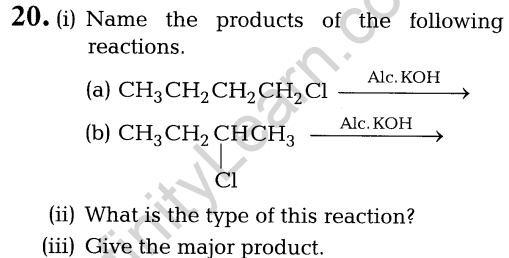
- oxidation number of iron.
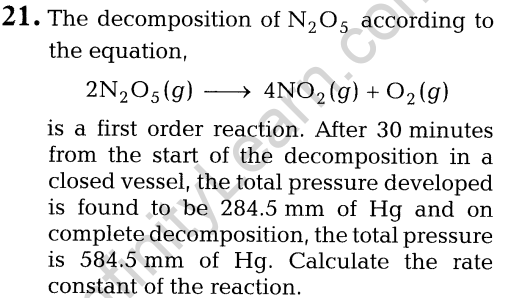
- the hybrid orbitals and the shape of the complex.
- the magnetic behaviour of the complex.
- the number of geometrical isomers.
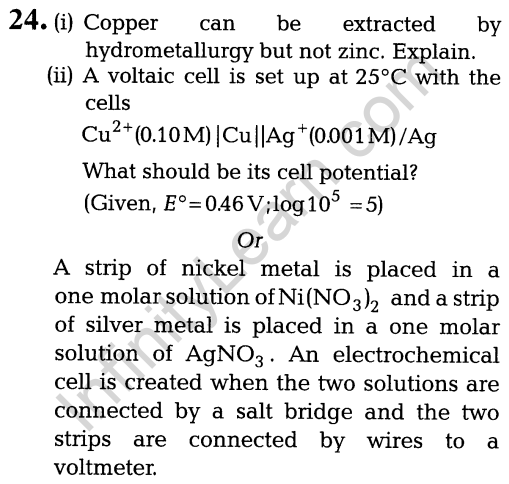
- whether there is an optical isomer.
- name of the complex.
15 (i) Write the two side effects of aspirin?
(ii) Explain the term with examples.
- Cationic detergents
- Anionic detergents
16. How are polymers classified into different categories on the basis of intermolecular forces? Give one example of a polymer of each of these categories.
17. Write the structural and functional differences between RNA and DNA.
Or
- What are the different types of RNA found in cell?
- What is the difference between nucleoside and nucleotide?
18. Convert the following:
- Phenol to aspirin.
- Salicylic acid to oil of wintergreen.
- Phenol to picric acid.
19 . (i) Why HF is stored in wax coated glass bottles?
- C1F3 exists but FC13 does not, why?
- Which compound formed when Xe reacts with PtF66 at
(a) 25°C? (b) 60°C?
22. What is an adsorption isotherm? Describe Freundlich adsorption isotherm.
Section D
23.In a chemical factory, ammonia was manufactured. A worker was about to open the knob of one of the chlorine cylinders one day for the fun but another co-worker stopped him vehemently to do so.
On the basis of above information, answer the following questions.
- Why did co-worker stopped him from opening the knob?
- Give the chemical reaction that would have occurred when the knob had been opened
- Write the chemical reaction involved for the preparation of ammonia in industries.
- Write some values exhibited by the co-worker.
Section E
25. Assign reasons for the following:
- The enthalpies of atomisation of transition elements are high.
- The transition metals and many of their compounds act as good catalyst.
- From element to element, the actinoid contraction is greater than the lanthanoid contraction.
- The E° value for the Mn3+ / Mn2+ couple is much more positive than that for Cr3+ / Cr2+ .
- Scandium (Z = 21) does not exhibit variable oxidation states and yet it is regarded as transition element.
Or
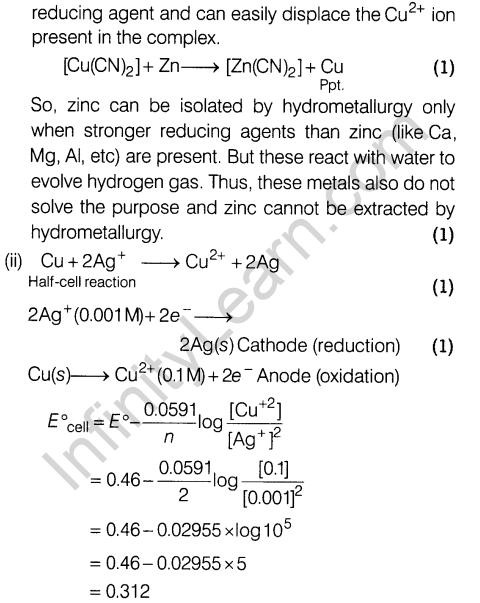
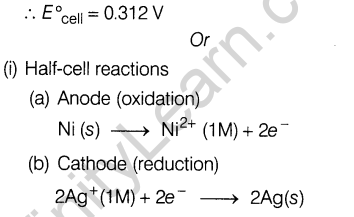
- How many unpaired electrons are present in Mn2+ ion (atomic number of Mn= 25)? How does it influence magnetic behaviour of Mn2+ ions?
- How would you account for the following?
- Metal-metal bonding is more extensive in the 4dand 5d series of transition elements than the 3 d series.
- Mn(III) undergoes disproportionation reaction easily.
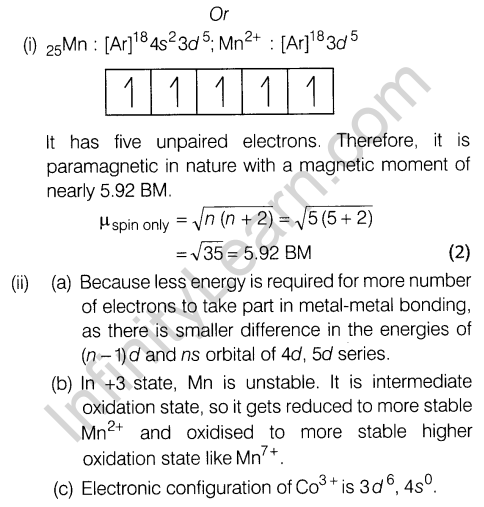
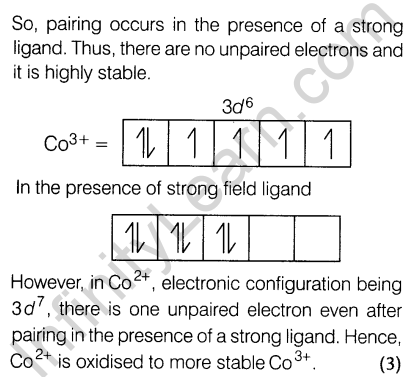
- Co(II) is easily oxidised in the presence of strong ligands.
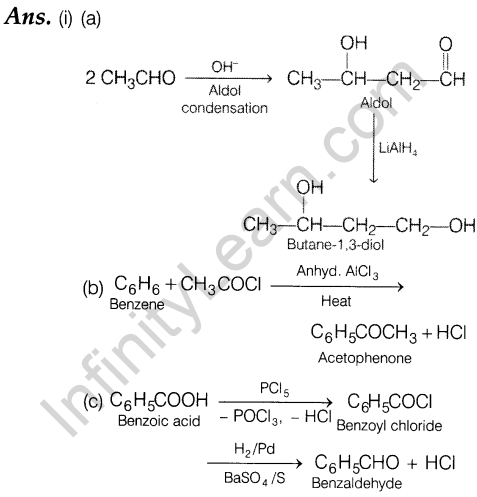
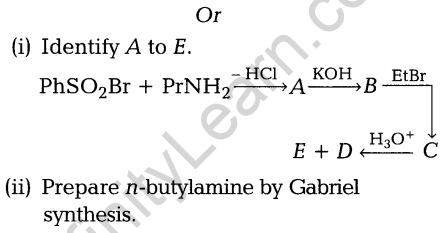
26. (i) How can the following conversions be carried out?
- Acetaldehyde to Butane-1,3-diol
- Benzene to acetophenone
- Benzoic acid to benzaldehyde (ii) Describe the following:
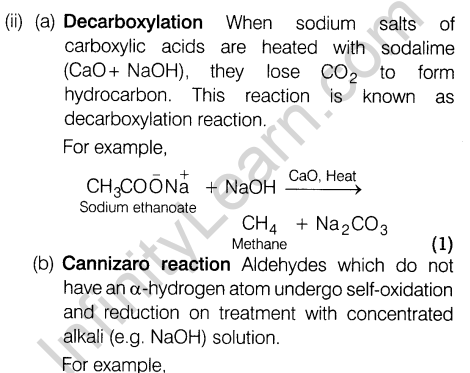
- Decarboxylation
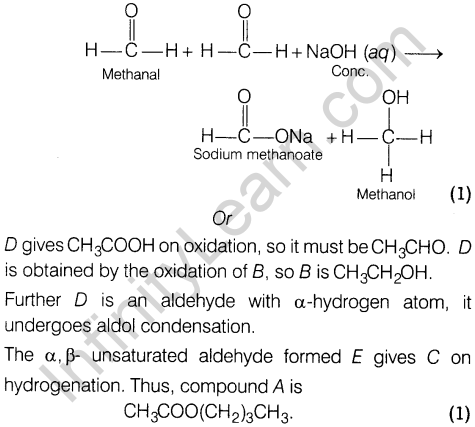
- Cannizzaro reaction
Or
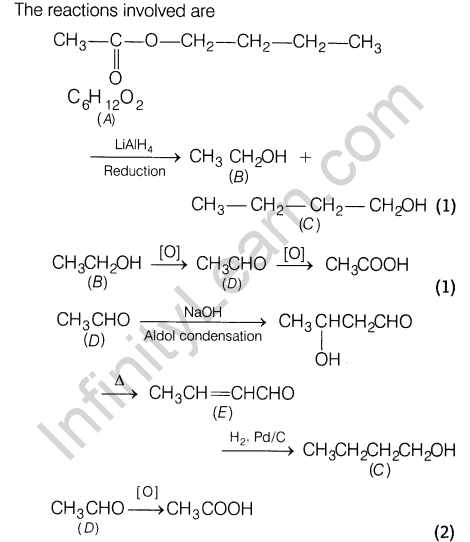
Compound A (C6H1202) on reduction with LiAlH4 yields two compounds B and C. The compound B on oxidation gave D which on treatment with aqueous alkali and subsequent heating furnished E.
Later on catalytic hydrogenation gave ‘C’. The compound ‘D’ on further oxidation gave CH3COOH. Deduce the structures of A, B, C, D and E.
Answers
Section A
1.How is dialysis carried out? Mention one application of it.
Ans.Dialysis is done by removing the excess of electrolyte from the colloidal solution, leaving behind a small amount of the electrolyte which accounts for its stability.
Application In case of kidney failure, harmful substances are removed from blood by dialysis
2.Name the process of heating sulphide ore in the presence of oxygen so as to convert it into oxide. Give one equation too.
Ans. Roasting.
![]()
3. Complete the following equation.

Ans.
4.How can you say that D-glucose is a reducing sugar?
Ans. D-glucose forms silver mirror with Tollen’s reagent and gives a red precipitate with Fehling’s solution, i.e. it reduces both the reagents. This shows that D-glucose is a reducing sugar.
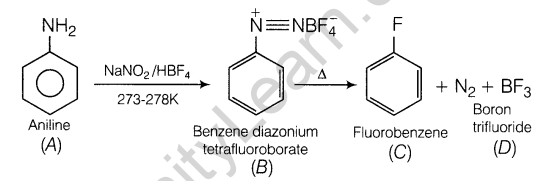
5.In automobile, there is ABS (Airbag System), which works on releasing a gas.
- Name the gas released.
- Provide the source
Ans. (i) N2 gas is released in pure state.
(ii) N2 in Airbag System of automobile can be formed by
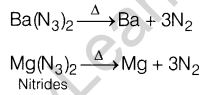
Section B
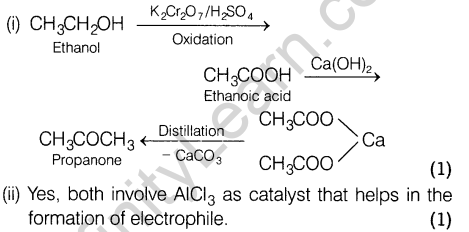
6. How will you convert ethanol to propanone?
(ii) Can Gattermann-Koch reaction be considered similar to Friedel-Crafts acylation?
Ans.

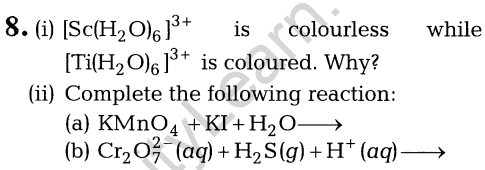



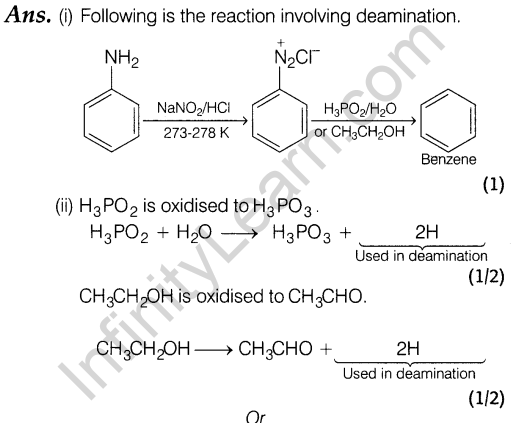
Section C
11.(i) How would you account for the following?
- Frenkel defects are not found in alkali metal halides.
- Schottky defects lower the density of related solids.
(ii) What happens when ferrimagnetic Fe3 04 is heated at 850 K and why?
Ans. (i) (a) Alkali metal cation is of large size and hence does not fit into the interstitial space. Thus, Frenkel defects is not found in these compounds.
(b) The presence of holes due to the missing of ions lowers the density of the crystal. (1)
(ii) Fe304 becomes paramagnetic.
Reason Because realignment of the magnetic movements or domains in one direction take place on heating.
12. (i) If the density of lake water is 1.25 g mL-1 and it contains 92 g of Na+ ions per kg of water, calculate the molality of Na+ ions in the lake.
(ii) At 300 K, 36 g of glucose present per litre in its solution has an osmotic pressure of 4.98 bar. If the osmotic pressure of the solution is 1.52 bar at the same temperature, what would be its concentration?
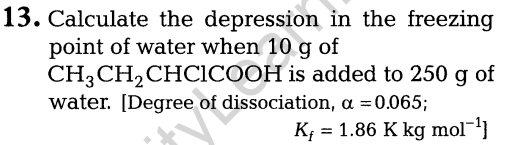
14. For the complex, [Fe(en)2Cl2]Cl (en = ethylene diamine), identify
- oxidation number of iron.
- the hybrid orbitals and the shape of the complex.
- the magnetic behaviour of the complex.
- the number of geometrical isomers.
- whether there is an optical isomer.
- name of the complex.
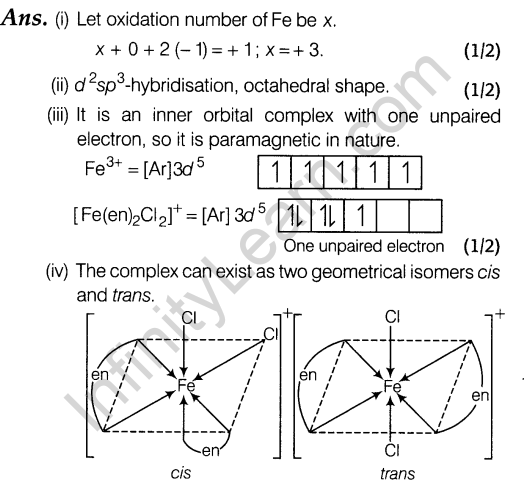
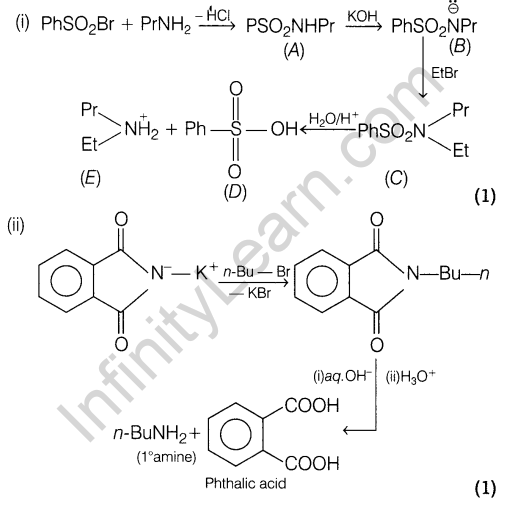
15 (i) Write the two side effects of aspirin?
(ii) Explain the term with examples.
- Cationic detergents
- Anionic detergents
Ans. Aspirin has two side effects, it is toxic to lever and it gets hydrolysed in the stomach to produce salicylic acid which causes bleeding from the stomach walls and thus, acts as gastric irritant and may produce ulcers.
(ii) (a) Cationic detergents These are quaternary ammonium salts of amines with acetates, chlorides or bromides.
Example, Cetyltrimethylammonium bromide
(b) Anionic detergents These detergents have large anionic part in their molecules. These are of two types.
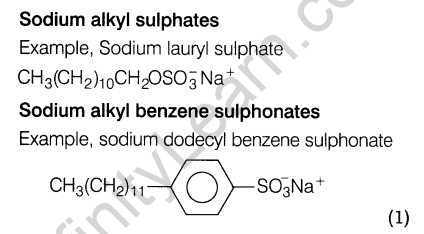
16. How are polymers classified into different categories on the basis of intermolecular forces? Give one example of a polymer of each of these categories.
Ans. Polymers are classified into the following four categories on the basis of magnitude of intermolecular forces present in them.
- Elastomers These are rubber-like solids with elastic properties. In these polymers, the polymer chains are held together by the weakest intermolecular forces. These weak binding forces permit the polymer to be stretched. A few crosslinks may also be present between the chains.
For example, buna-S, buna-N, neoprene, etc.
- Fibres These fibres are thread-forming solids with high tensile strength and high modulus. The intermolecular forces (e.g. H-bonding, etc.) are very strong so chains are closely packed with crystalline nature.
For example, polyamides like nylon-66 and polyesters like terylene, etc.
- Thermoplastic polymers These are linear or slightly branched long chain molecules which soften on heating and harden on cooling. Their intermolecular forces of attraction are intermediate between elastomers and fibres.
For example, polythene, polystyrene, polyvinyls, etc.
- Thermosetting polymers These polymers are cross linked or heavily branched molecules, which on heating undergo extensive cross linking in moulds and again become infusible. These cannot be remoulded.
For example, bakelite, urea-formaldehyde resins, etc.
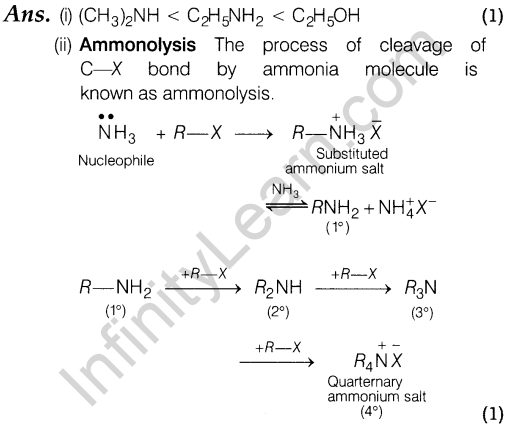
17. Write the structural and functional differences between RNA and DNA.
Or
- What are the different types of RNA found in cell?
- What is the difference between nucleoside and nucleotide?
Ans. Structural differences between DNA and RNA are
- In DNA, the pentose sugar is D-2-deoxyribose but in RNA, it is D-ribose.
- The pyrimidine bases in DNA are cytosine and thymine while in RNA, these are cytosine and uracil.
- DNA has double stranded a-helix structure while, RNA has single stranded a-helix structure.
- Molecular mass of DNA is higher than that of RNA.
Functional differences between DNA and RNA are
- DNA has unique property of replication while, RNA generally does not have.
- DNA controls the transmission of hereditary characters
while, RNA controls protein synthesis.
Or
- Three types of RNA present in the cell are
- Ribosomal RNA (r-RNA)
- Messenger RNA (m-RNA)
- Transfer RNA (f-RNA) (1)
- The differences between nucleoside and nucleotide are
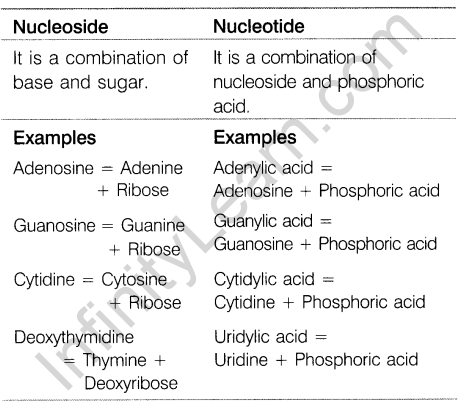
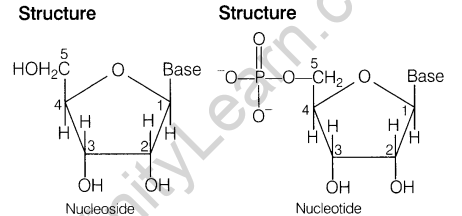
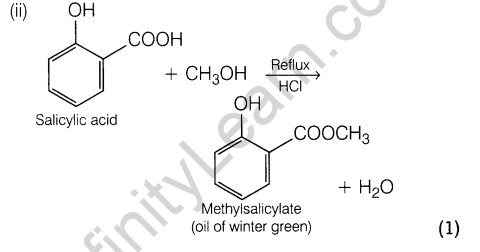
18. Convert the following:
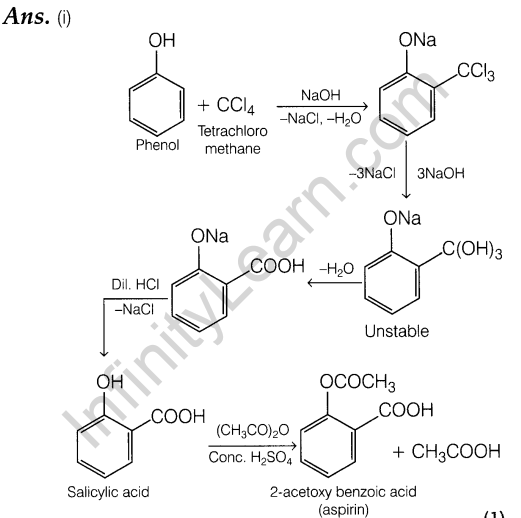
- Phenol to aspirin.
- Salicylic acid to oil of winter green.
- Phenol to picric acid.
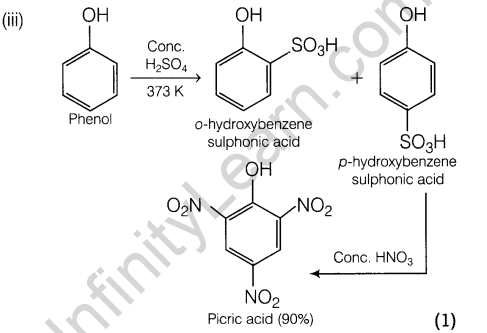
19 . (i) Why HF is stored in wax coated glass bottles?
- C1F3 exists but FC13 does not, why?
- Which compound formed when Xe reacts withPtF6 at
(a) 25°C? (b) 60°C?
Ans. (i) HF does not attack wax but reacts with glass. It dissolves Si02 present in glass forming hydrofluorosilicic acid.
Si02+ 6HF——— > H2SiF6+ 2H20
That’s why, it is stored in wax coated glass bottles.
(ii) Fluorine is more electronegative than chlorine and much smaller in size, so it cannot hold three large sized Cl-atoms.
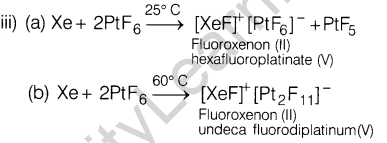

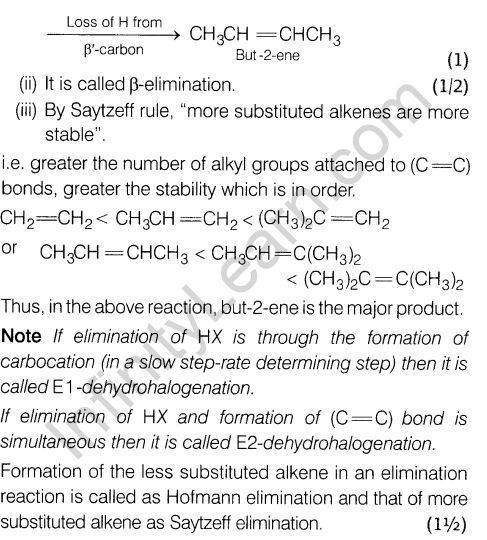

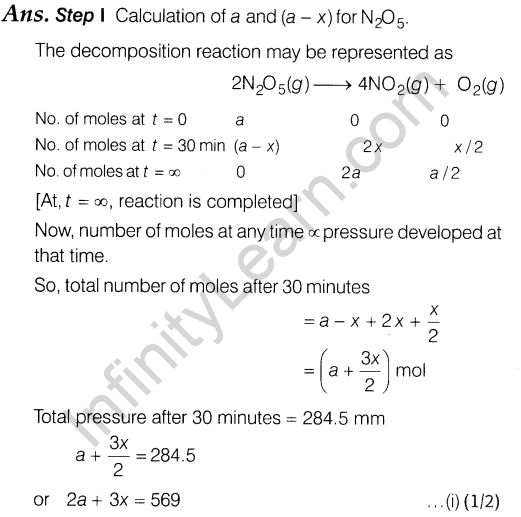
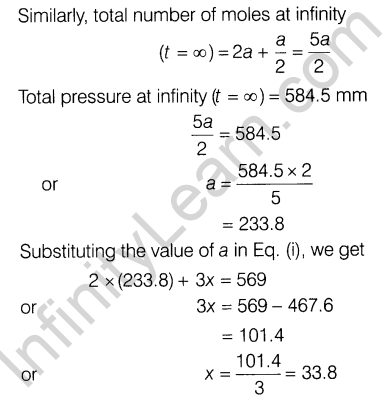
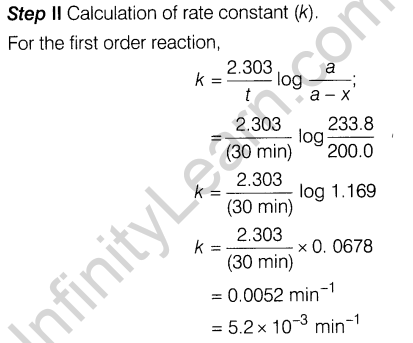
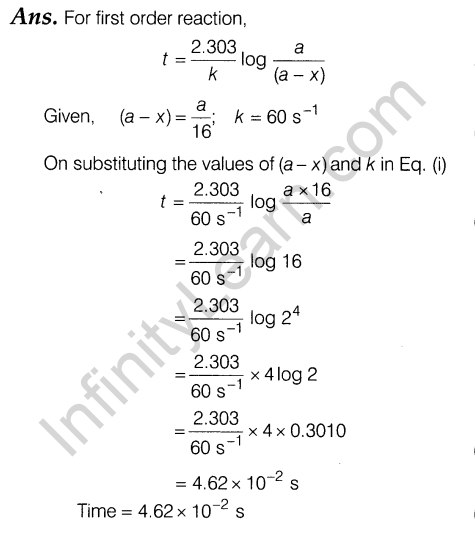
5.2 x 10~3 min-1
22. What is an adsorption isotherm? Describe Freundlich adsorption isotherm.
Ans. Adsorption isotherm The variation in the amount of gas adsorbed by the adsorbent with pressure at constant temperature can be expressed by means of a curve known as adsorption isotherm.
Adsorption isotherms are of two types :
- Freundlich adsorption isotherm
- Langmuir adsorption isotherm (1)
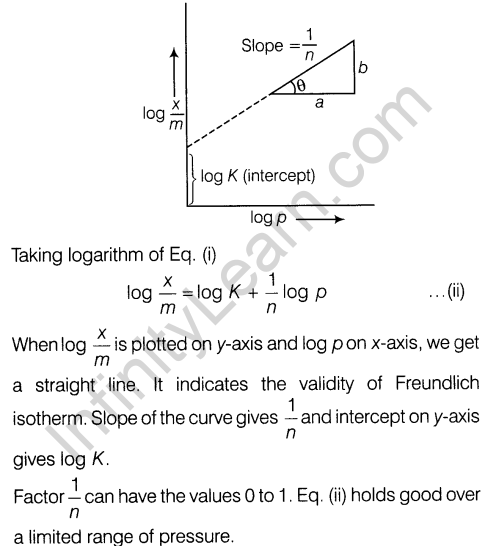
Freundlich adsorption isotherm Freundlich gave an empirical relationship between the quantity of gas adsorbed by unit mass of solid adsorbent and pressure at a particular temperature.
The relationship is expressed by the given equation

where, x is the mass of the gas adsorbed on mass m of the adsorbent at pressure p. K and n are the constants which depend on the nature of the adsorbent and the gas at a particular temperature.
Graph showing adsorption isotherm is shown below
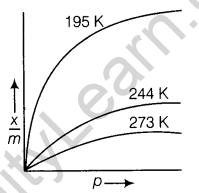
These curves indicate that at a fixed pressure, there is a decrease in physical adsorption with increase in temperature.
Graph showing Freundlich isotherm is shown below.

Section D
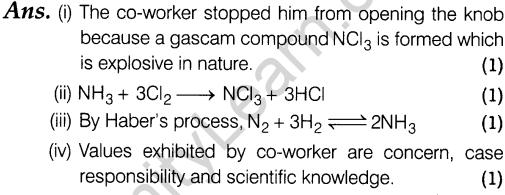
23.In a chemical factory, ammonia was manufactured. A worker was about to open the knob of one of the chlorine cylinders one day for the fun but another co-worker stopped him vehemently to do so.
On the basis of above information, answer the following questions.
- Why did co-worker stopped him from opening the knob?
- Give the chemical reaction that would have occurred when the knob had been opened
- Write the chemical reaction involved for the preparation of ammonia in industries.
- Write some values exhibited by the co-worker.
Section E



25. Assign reasons for the following:
- The enthalpies of atomisation of transition elements are high.
- The transition metals and many of their compounds act as good catalyst.
- From element to element, the actinoid contraction is greater than the lanthanoid contraction.
- The E° value for the Mn3+ / Mn2+ couple is much more positive than that for Cr3+ / Cr2+ .
- Scandium (Z = 21) does not exhibit variable oxidation states and yet it is regarded as transition element.
Or
- How many unpaired electrons are present in Mn2+ ion (atomic number of Mn= 25)? How does it influence magnetic behaviour of Mn2+ ions?
- How would you account for the following?
- Metal-metal bonding is more extensive in the 4dand 5d series of transition elements than the 3 d series.
- Mn(III) undergoes disproportionation reaction easily.
- Co(II) is easily oxidised in the presence of strong ligands.
Ans. (i) Transition elements have high enthalpies of atomisation due to strong metallic bonding and additional covalent bonding. Metallic bonding is due to their smaller size while covalent bonding is due to d-d overlapping.
(ii) The catalytic activity of the transition elements can be explained by two basic facts. Firstly, owing to their ability to show variable oxidation states and form complexes. Transition metals form unstable intermediate compounds. Thus, they provide a new path by lowering the activation energy, Ea, for the reaction and secondly transition metals also provide a suitable surface for the reactions to occur.
(iii) Because 5f subshell is more diffused than 4f-subshell, the shielding by 5f electrons in actinoids is much less than by 4f electrons in lanthanoids. Therefore, magnitude of actinoid contraction is much more than that of lanthanoid contraction.
(iv) The comparatively high value for Mn is due to the fact that Mn2+(d5) is particularly stable whereas comparatively low value for Cr is because of the extra stability of Cr3+(d 3) as t2g is partially filled (i.e. t2g3,eg). Therefore, Cr3+ cannot be reduced to Cr2+.
(v) Transition elements are those elements that have partially filled d-subshell in their elementary form or in their commonly occurring oxidation state.
As scandium has partially filled d-subshell in its elementary form, it is considered as a transition element.
21Sc= [Ar]184s23d1
26. (i) How can the following conversions be carried out?
- Acetaldehyde to Butane-1,3-diol
- Benzene to acetophenone
- Benzoic acid to benzaldehyde (ii) Describe the following:
- Decarboxylation
- Cannizzaro reaction
Or
Compound A (C6H1202) on reduction with LiAlH4 yields two compounds B and C. The compound B on oxidation gave D which on treatment with aqueous alkali and subsequent heating furnished E.
Later on catalytic hydrogenation gave ‘C’. The compound ‘D’ on further oxidation gave CH3COOH. Deduce the structures of A, B, C, D and E.