Table of Contents
Time allowed: 3 hours
Maximum Marks: 70
CBSE Previous Year Question Papers Class 12 Chemistry 2013 Delhi Set I
Question 1.
How many atoms constitute one unit cell of a face-centred cubic crystal? [1]
Question 2.
Name the method used for refining of Nickel metal. [1]
Answer:
The method used for refining of nickel metal is Mond’s process.
Question 3.
What is the covalency of nitrogen in N2O5? [1]
Question 4.
Write the IUPAC name of: [1]
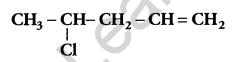
Answer:
4-chloropent-1-ene.
Question 5.
What happens when CH3Br is treated with KCN? [1]
Answer:
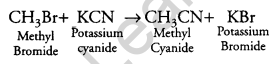
It is a nucleophilic substitution reaction.
Question 6.
Write the structure of 3-methyl butanal. [1]
Answer:
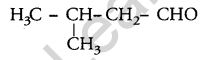
Question 7.
Arrange the following in increasing order of their basic strength in aqueous solution:
CH3NH2, (CH3)3N, (CH3)2NH. [1]
Answer:

Question 8.
What are the types of an RNA molecule which perform different functions? [1]
Answer:
Three types of RNA molecules which perform different functions are:
- Messenger RNA (m-RNA)
- Transfer RNA (t-RNA)
- Ribosomal RNA (r-RNA)
Question 9.
18 g of glucose, C6H12O6 (Molar Mass = 180g/ mol) is dissolved in 1 kg of water in a saucepan. At what temperature will this solution boil? [2] (Kb for water = 0.52 K kg mol-1, boiling point of pure water = 373.15 K)
Answer:
Given,
Weight of solvent (H2O) (W1) = 1 kg
Weight of solute (C6H12O6) (W2) = 18 gm
Molar mass of solute (M2) = 180 g/mol
Kb = 0.52 K kg mol-1
T0b = 373.15 K
We know that
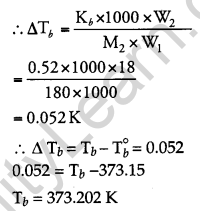
Hence, the boiling point of a solution is 373.202 K
Question 10.
The conductivity of 0.20 M solution of KCl at 298 K is 0.025 S cm-1. Calculate its molar conductivity. [2]
Answer:
Given k = 0.025 S cm-1
Molarity, M = 0.20 M
Molar conductivity
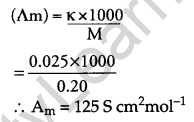
Question 11.
Write the dispersed phase and dispersion medium of the following colloidal system: [2]
(i) Smoke
(ii) Milk.
OR
What are lyophilic and lyophobic colloids? Which of these sols can be easily coagulated on the addition of small amounts of electrolytes?
Answer:
(i) Dispersed Phase in Smoke: Solid
Dispersion medium in smoke: Gas
(ii) Dispersed Phase in Milk: Fats (liquid)
Dispersion medium in milk: Water (liquid)
OR
Lyophilic Colloids: These are the colloidal solutions in which the dispersed phase has a great affinity for the dispersion medium. Such solutions are quite stable and are reversible in nature, e.g. starch, proteins, etc.
Lyophobic Colloids (Liquid Hating): These are the colloidal solutions in which dispersed phase has very little affinity for the dispersion medium. Such solutions are unstable and are irreversible in nature, eg., (AS2S3 Sol).
Lyophobic colloids can be easily coagulated because, on the addition of the small amount of electrolyte, the charge on colloidal particles is removed, as a result, the particles will come closer to each other and then aggregate to form a cluster which settles down under the force of gravity.
Question 12.
Write the differences between physisorption and chemisorption with respect to the following: [2]
(i) Specificity
(ii) Temperature dependence
(iii) Reversibility and
(iv) Enthalpy change Answer:
Answer:
|
S.No. |
Point of difference | Physisorption | Chemisorption |
| (i) | Specificity | It is not specific in nature i.e., all gases are adsorbed on all solids to some extent. | This is highly specific in nature. i.e., it occurs only when there is some possibility of compound formation between the gas being adsorbed and the solid being adsorbent. |
| (ii) | Temperature dependence | Low temperature is favourable for physisorption. It decreases with increase in temperature | High temperature is favourable for chemisorptions. It increases with the increase in temperature. |
| (iii) | Reversibility | It is reversible in nature. | It is irreversible in nature. |
| (iv) | Enthalpy change | Enthalpy of adsorption is low i.e., 20-40 kJ mol-1 | Enthalpy of adsorption is high i.e., 404000 kJ mol-1 |
Question 13.
(a) Which solution is used for the leaching of Silver metal in the presence of air in the metallurgy of silver?
(b) Out of ‘C’ and ‘CO’, which is a better reducing agent at the lower temperature range in the blast furnace to extract iron from the oxide ore? [2]
Answer:
(a) Dilute solution i.e., 0.5% NaCN and KCN is used for leaching of silver metal in the presence of air.
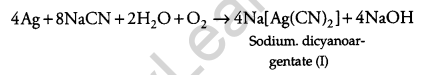
(b) Out of C and CO, CO is a better reducing agent at a lower temperature range in the blast furnace to extract iron from the oxide ore because in Ellingham diagram ΔG(CO, CO2) < ΔG(Fe, FeO) SO, CO will reduce FeO to Fe and will be oxidized to CO2.
![]()
Question 14.
What happens when [2]
(i) PCl5 is heated?
(ii) H3PO3 is heated?
Write the reaction involved.
Answer:
(i) PCl5 on heating gives PCl3 and Cl2.
![]()
(ii) H3PO3 on heating gives orthophosphoric acid and phosphine.
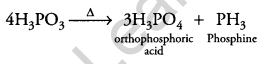
Question 15.
(a) Which metal in the first transition series (3d series) exhibits +1 oxidation state most frequently and why?
(b) Which of the following cations are coloured in aqueous solutions and why? [2]
Sc3+, V3+, Ti4+, Mn2+
(Atomic Nos. Sc = 21, V = 23, Ti = 22, Mn = 25)
Answer:
(a) Cu is the only metal in the first transition series (3d series) which exhibits +1 oxidation state more frequently. This is because the electronic configuration of Cu is 3d10 4s1 and after losing ones electron it acquires a stable 3d10 configuration.
(b) The colour of cations depends upon the number of impaired electrons present in d-orbital. The electronic configuration of the following cations is as follows:
Sc (Atomic number 21) = 3d14s2 and Sc3+ = 3d04s0.
As d-orbital is empty, it is colourless.
V (atomic number 23) = 3d34s2 and V3+ = 3d24s0.
As d-orbital is having 2 impaired electrons, it undergoes d-d transition and depicts green colour.
Ti (Atomic number 22) = 3d24s2 and Ti4+ = 3d04s0.
As ‘d’ orbital is empty, it is colourless.
Mn(Atomic Number 25) = 3d54s2 and Mn2+ = 3d54s0.
As ‘d’ orbital has 5 unpaired electrons, it depicts a pink colour.
Thus, V3+ and Mn2+ ions are coloured in their aqueous solution due to the presence of impaired electron.
Question 16.
Chlorobenzene is extremely less reactive towards a nucleophilic substitution reaction. Give two reasons for the same. [2]
Answer:
Chlorobenzene is extremely less reactive towards a nucleophilic substitution reaction because of the following reasons:
1. Resonance Effect: The electron pair on chlorine atom is in conjugation with the benzene electrons of the benzene ring which results in the following resonating structures:
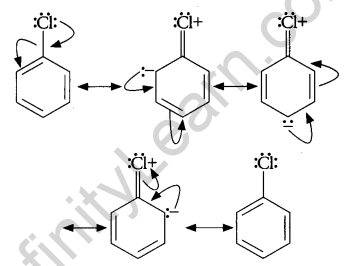
This results in delocalization of electrons of C-Cl bond and a partial double bond character develops in the bond, which makes it difficult for the nucleophile to cleave the C-Cl bond.
2. The nucleophile suffers repulsion from the increased electron density on the benzene ring, as a result, the nucleophile is unable to make a close approach for the attack on the molecule.
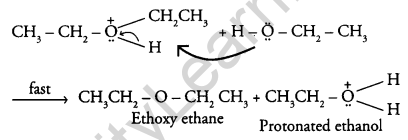
Question 17.
Explain the mechanism of the following reaction: [2]
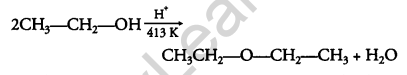
Answer:
The mechanism of the reaction is given below:

Question 18.
How will you convert:
(i) Propene to Propan-2-ol?
(ii) Phenol to 2, 4, 6 – trinitrophenol?
Answer:
(i) When H2SO4 is added to propene, propan-2-ol is formed. The addition of H2SO4 takes place in accordance with Markovnikov’s rule.
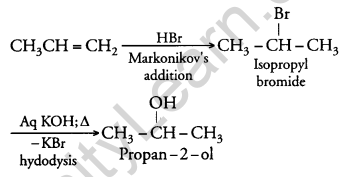
(ii) When concentrated nitric acid is added to phenol in the presence of sulphuric acid it gives 2, 4, 6 – trinitrophenol.
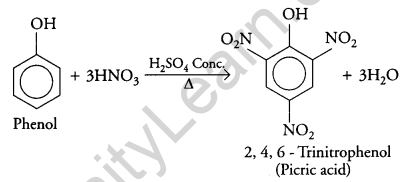
Question 19.
(a) What type of semiconductor is obtained when silicon is doped with boron? [3]
(b) What type of magnetism is shown in the following alignment of magnetic moments?

(c) What type of point defect is produced when AgCl is doped with CdCl2?
Question 20.
Determine the osmotic pressure of a solution prepared by dissolving 2.5 × 10-2 g of K2SO4 in 2 L of water at 25°C, assuming that it is completely dissociated. [3]
(R = 0.0821 L atm K-1 mol-1, Molar mass of K2SO4 = 174 g mol-1)
Answer:
We know
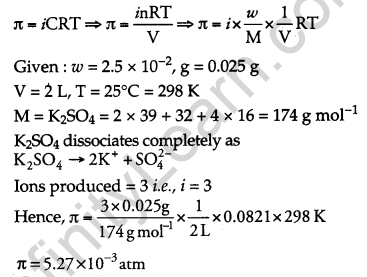
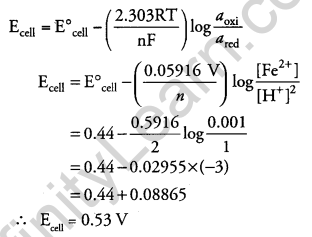
Question 21.
Calculate the emf of the following cell at 298 K: [3]
Fe(s) | Fe2+ (0.001 M) || H+(1M) | H2(g) (1 bar), Pt(s)
(Given E0cell = +0.44 V)
Answer:
At anode : Fe → Fe2+ + 2e–
At cathode: 2H+ + 2e– → H2
So, total number of electrons (n) transferred = 2
Given that: E0cell = +0.44 V
Temperature (T) = 298 K
We know,
Question 22.
How would you account for the following? [3]
(i) Transition metals exhibit variable oxidation states.
(ii) Zr (Z = 40) and Hf (Z = 72) have almost identical radii.
(iii) Transition metals and their compounds act as a catalyst.
OR
Complete the following chemical equations:
(i) Cr2O2-7 + 6Fe2+ + 14H+ →
(ii) 2CrO4–+ 2H+ →
(iii) 2MnO–4 + 5C2O-24 + 16H+ →
Answer:
(i) The variable oxidation states of transition elements are due to the participation of ns and (n – 1)d-electrons in bonding. Lower oxidation state is exhibited when ns-electrons take part in bonding. Higher oxidation states are exhibited when (n – 1) d-electrons take part in bonding.
(ii) This is because the atomic radii of 4d and 5d transition elements are nearly the same. This similarity in size is a consequence of lanthanide contraction which is due to weak shielding of d-electrons. As a result, the radii of Hf becomes nearly equal to that of Zr.
(iii) The catalytic activity of transition elements and their compound is due to the following reasons:
1. Due to their tendency to show variable oxidation state transition metal form unstable intermediate compounds and provides a new path for the reaction with lower activation energy.
2. In some cases, the transition metals provide a suitably large surface area with free valencies or ion which reactant can adsorb.
OR
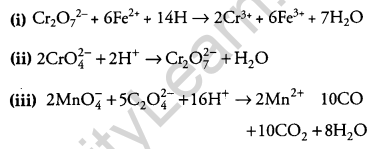
Question 23.
Write the IUPAC names of the following coordination compounds: [3]
(i) [Cr(NH3)3Cl3]
(ii) K3[Fe(CN)6]
(iii) [CoBr2(en)2]+, (en = ethylenediamine)
Answer:
(i) Triamminetrichlorochromium(III)
(ii) Potassium hexacyanoferrate(III)
(iii) Dibromobis-(ethylenediamine) cobalt (III) ion
Question 24.
Give the structures of A, B and C in the following reactions: [3]
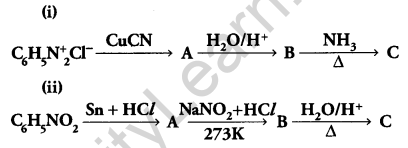
Answer:
(i)
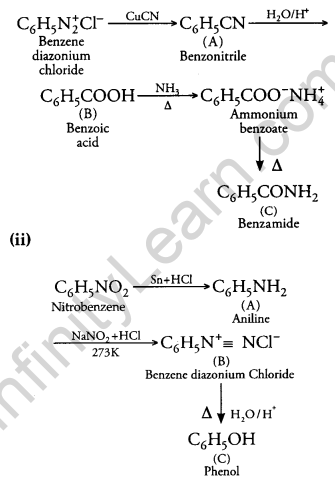
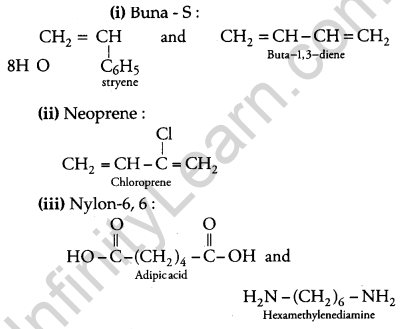
Question 25.
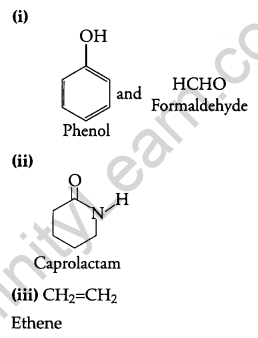
Write the names and structures of the monomers of the following polymers: [3]
(i) Buna-S
(ii) Neoprene
(iii) Nylon-6, 6
Answer:
Question 26.
After watching a programme on TV about the adverse effects of junk food and soft drinks on the health of school children, Sonali, a student of Class XII, discussed the issue with the principal. The principal immediately instructed the canteen contractor to replace the fast food with the fibre and vitamins rich food like sprouts, salad, fruits etc. This decision was welcomed by the parents and the students. [3]
After reading the above passage, answer the following questions:
(a) What values are expressed by Sonali and the Principal of the school?
(b) Give two examples of water-soluble vitamins.
Answer:
(b) The two water-soluble vitamins are vitamin B and Vitamin C (Ascorbic acid).
Question 27.
- Which one of the following is a food preservative? [3] Equanil, Morphine, Sodium benzoate
- Why is bithional added to soap?
- Which class of drugs is used in sleeping pills?
Answer:
- Sodium benzoate is used as a food preservative whereas equanil is a tranquiliser and morphine is a narcotic analgesic.
- Bithional is an antiseptic so it is added to soaps to reduce the odours produced by bacterial decomposition of organic matter on the skin.
- Tranquilisers relieve stress, fatigue by inducing a sense of well being, so they are used in sleeping pills.
Question 28.
(a) A reaction is second order in A and first order in B. [5]
(i) Write the differential rate equation.
(ii) How is the rate affected increasing the concentration of A three times?
(iii) How is the rate affected when the concentrations of both A and B are doubled?
(b) A first-order reaction takes 40 minutes for 30% decomposition. Calculate t1/2 for this reaction.
(Given: log 1.428 = 0.1548)
OR
(a) For a first-order reaction, show that time required for 99% completion is twice the time required for the completion of 90% of the reaction.
(b) Rate constant ‘k’ of a reaction varies with temperature ‘T’ according to the equation:
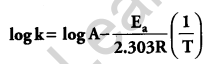
Where Ea is the activation energy. When a graph is plotted for log k Vs. \(\frac { 1 }{ T }\), a straight line with a slope of -4250 K is obtained. Calculate ‘Ea‘ for the reaction. (R = 8.314 JK-1 mol-1)
Answer:
(a) (i) A reaction is second order in A and first order in B.
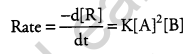
(ii) On increasing the concentration of ‘A’ three times i.e. 3A; the rate of reaction becomes 9 times the initial rate.
Rate = K[3A]2[B] = 9K[A]2[B] = 9 times rate.
(iii) On increasing the concentration of A and B as 2A and 2B. The rate of reaction becomes 8 times the initial rate.
Rate = K[2A]2[2B] = 8K[A]2[B] = 8 times rate
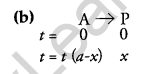
Now, it takes 40 min for 30% decomposition i.e. reactant left after 40 min is 70% of its initial concentration.

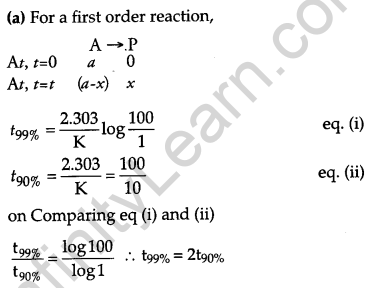
the time required for 99% completion of 1st order reaction is twice the time required for 90% completion.
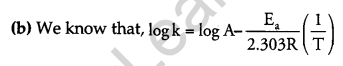
The above equation is like y = mx + c, where if we plot ‘y’ vs ‘x’ we get a straight line with slope ‘m’ and intercept ‘c’.
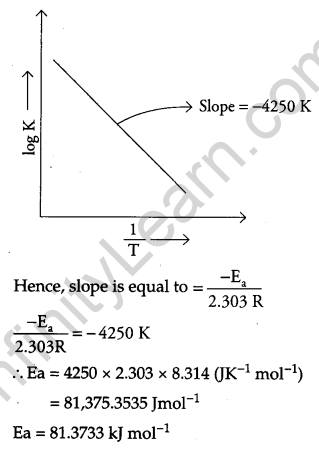
Question 29.
(a) Give reasons for the following: [5]
(i) Bond enthalpy of F2 is lower than that of Cl2.
(ii) PH3 has a lower boiling point than NH3.
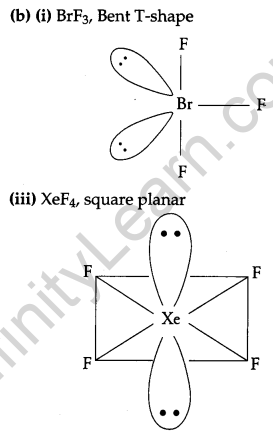
(b) Draw the structures of the following molecules:
(i) BrF3
(ii) (HPO3)3
(iii)XeF4
OR
(a) Account for the following:
(i) Helium is used in diving apparatus.
(ii) Fluorine does not exhibit a positive oxidation state.
(iii) Oxygen shows catenation behaviour less than sulphur.
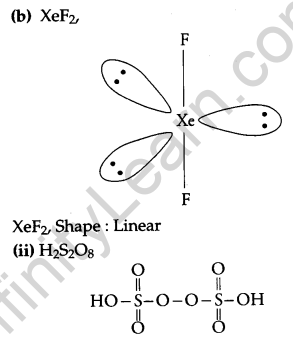
(b) Draw the structures of the following molecules.
(i) XeF2
(ii) H2S2O8
Answer:
(a) (i) Bond enthalpy of F2 is lower than that of Cl2 because ‘F’ atom is small in size and due to this the electron-electron repulsions between the lone pairs of F-F electrons are very large. Thus, the bond dissociation energy of F2 is lower than that of Cl2.
OR
(a) (i) Helium mixed with oxygen under pressure is given to sea-divers for artificial respiration because of its very low solubility in blood. Air is not given because nitrogen present in air being soluble in blood will give a painful sensation called bends by bubbling out blood on moving from high pressure to the atmospheric pressure. Thus, the oxygen-helium mixture is used.
(ii) Fluorine being the most electronegative atom does not exhibit positive oxidation state because, it does not have d-orbitals for octet expansion and therefore, it shows only a negative oxidation state of -1.
(iii) Oxygen shows catenation behaviour less than sulphur because the oxygen atom is smaller in size as compared to sulphur due to this the lone pair of an electron in 0-0 bonds in oxygen experiences more repulsion as compared to the S-S bonds and thus, S-S forms a strong bond.
Question 30.
(a) Although phenoxide ion has a number of resonating structures than carboxylate ion, the carboxylic acid is a stronger acid than phenol. Give two reasons. [5]
(b) How will you bring about the following conversions?
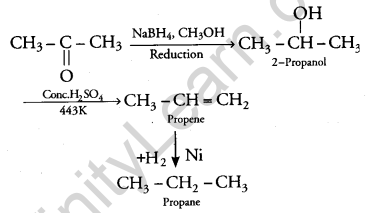
(i) Propanone to propane
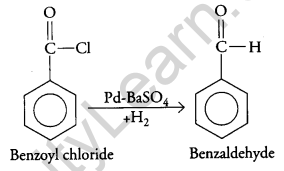
(ii) Benzoyl chloride to benzaldehyde
(iii) Ethanal to but-2-enal.
OR
(a) Complete the following reactions:
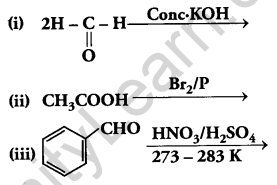
(b) Give simple chemical tests to distinguish between the following pairs of compounds:
(i) Ethanal and Propanal
(ii) Benzoic acid and Phenol.
Answer:
(a) On losing a proton, carboxylic acids form carboxylate ion and phenol forms phenoxide ion as follows:
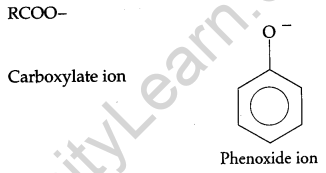
Now, the negative charge is delocalized in both molecules as follows:
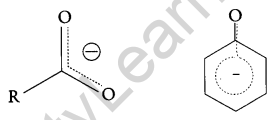
The conjugate base of carboxylic acid has two resonance structures in which negative charge is delocalized over two oxygen atoms which stabilize the carboxylate ion.
On the other hand, in phenoxide ion the charge is delocalized over the entire molecule on the less electronegative atom, thus resonance of phenoxides is not important in comparison to resonance in carboxylate ion.
Further, in carboxylate ion, the negative charge is effectively delocalized over two oxygen atoms whereas it is less effectively delocalized over one oxygen atom and a less electronegative carbon atom.
Thus, phenol is less acidic than carboxylic acids. In other words, carboxylic acids are stronger acids than phenol.
(b) (i) Conversion of propanone to propane:
(ii) Conversion of benzoyl chloride to benzaldehyde:
(iii) On treatment with dilute alkali, ethanol produces 3-hydroxybutanal gives But-2-enal on heating
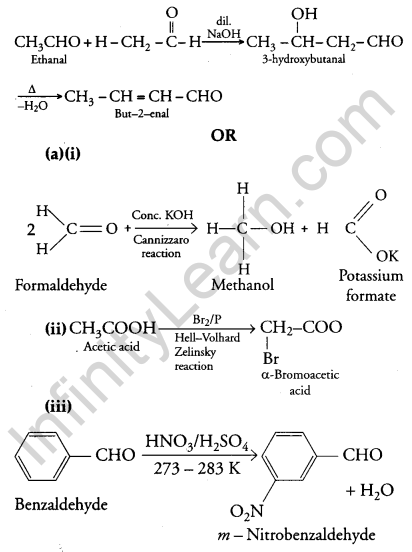
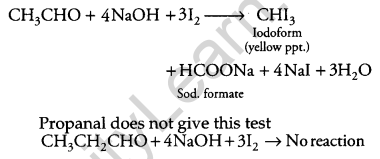
(b) (i) Ethanol and Propanal:
Iodoform Test: When ethanol is treated with sodium hydroxide and ammonia, iodoform is obtained.
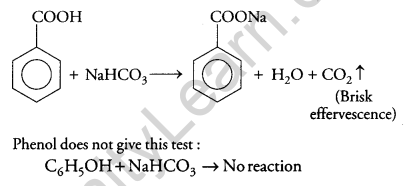
(ii) Benzoic acid and phenol:
NaHCO3 Test: When benzoic acid is treated with NaHCO3 brisk effervescence of CO2 gas was evolved.
CBSE Previous Year Question Papers Class 12 Chemistry 2013 Delhi Set II
Note: Except for the following questions, all the remaining questions have been asked in the previous set.
Question 1.
What type of stoichiometric defect is shown by AgCl? [1]
Question 2.
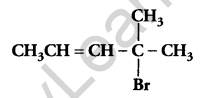
Write the IUPAC name of [1]
Answer:
4-Bromo-4-methylpent-2-ene
Question 4.
What type of bonding helps in stabilizing the a-helix structure of proteins? [1]
Answer:
Hydrogen bonding between the NH group of each amino acid residue and the >C = O of an adjacent turn of the helix helps in stabilizing the α-helix structure of proteins.
Question 6.
What inspired N.Bartlett for carrying out a reaction between Xe and PtF6? [1]
Answer:
N.Bartlett observed that the first ionization enthalpy of molecular oxygen is almost identical to that of xenon. So after preparing red coloured compound O2PtF62 he made efforts to prepare Xe+PtF–6 by mixing PtF6 and Xe.
Question 7.
What happens when ethyl chloride is treated with aqueous KOH? [1]
Answer:
When C2H5Cl reacts with aq. KOH, substitution Nucleophilic bimolecular (SN2) reaction takes place and Ethanol is formed.
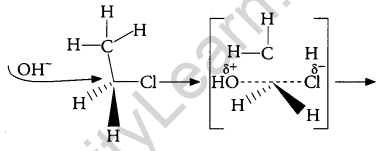
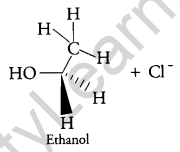
And inversion of configuration also takes place.
Question 8.
Write the structure of 4-chloropentan-2-one. [1]
Answer:
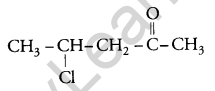
Question 9.
How will you convert the following?
(i) Propan-2-ol to propanone.
(ii) Phenol to 2,4,6 – tribromophenol?
Answer:
(i) Propan-2-ol to propanone:
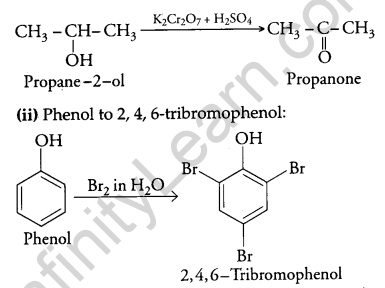
Question 11.
What is the difference between oil/water (O/W) type and water/oil (W/O) type emulsions? Give an example of each type. [2]
Answer:
Emulsion of oil-in-water has oil as dispersed phase and water as the dispersion medium. For example, Milk etc.
Emulsion of water-in-oil has water as dispersed phase and oil as the dispersion medium. For example, Cod liver oil etc.
Question 17.
(a) Which of the following ores can be concentrated by froth floatation method and why?
Fe2O3, ZnS, Al2O3
(b) What is the role of silica in the metallurgy of Copper? [2]
Answer:
(a) Only sulphide ores are concentrated by this process because pine oil selectively wets the sulphide ore and hence bring it to the froth.
(b) Silica is added in the reverberatory furnace during the extraction of copper to remove iron oxide present in the ore. Iron oxide reacts with the silica and is removed as a slag of iron silicate.
FeO + SiO2 → FeSiO3 (Slag)
Question 18.
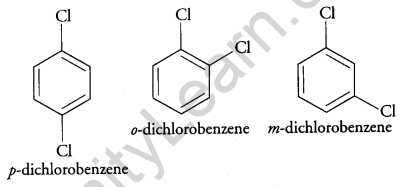
(a) Why does p-dichlorobenzene have a higher m.p. than its o- and m- isomers? [2]
(b) Why is (-) – Butan-2-ol optically inactive?
Answer:
(a) p-dichlorobenzene has a higher melting point than ortho and meta isomer. This is because the para isomer is having a symmetrical structure and therefore, its packing is more efficient as compared to the ortho and meta isomer, therefore, it shows a higher melting point.
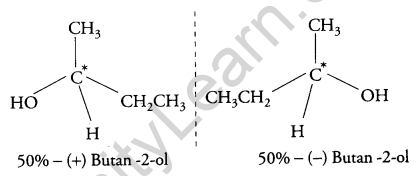
(b) The (±) – Butan-2-ol is optically inactive because it is a racemic mixture and exists in two enantiomeric forms which are non- superimposable mirror images of each other. Both the isomers are present in equal amounts therefore, it does not rotate the plane of polarized light and is optically inactive.
Question 23.
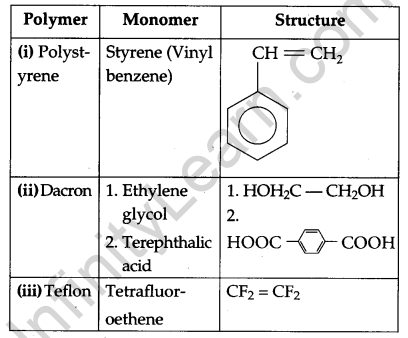
Write the names and structures of the monomers of the following polymers: [3]
(i) Polystyrene
(ii) Dacron
(iii) Teflon
Answer:
Polymers with their monomers and their structures:
Question 27.
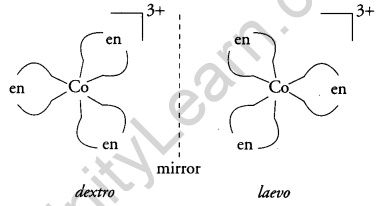
Write the types of isomerism exhibited by the following complexes: [3]
(i) [CO(NH3)5Cl]SO4
(ii) [Co(en)3]3+
(iii) [CO(NH3)6] [Cr(CN)6]
Answer:
(i) Ionisation isomerism
(ii) Optical isomerism
(iii) Coordination isomerism
CBSE Previous Year Question Papers Class 12 Chemistry 2013 Delhi Set III
Note: Except for the following questions, all the remaining questions have been asked in previous sets.
Question 1.
What type of substances would make better Permanent Magnets, Ferromagnetic or Ferrimagnetic? [1]
Question 3.
What is the composition of ‘Copper matte’? [1]
Answer:
Composition of ‘Copper matte’ is Cu2S and FeS.
Question 5.
What is a glycosidic linkage? [1]
Answer:
The linkage between the two monosaccharide units through oxygen atom accompanied by the loss of a water molecule is called glycosidic linkage.
Question 6.
Write the IUPAC name of (CH3)2CH.CH(Cl)CH3 [1]
Answer:
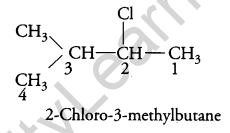
Question 7.
Which compound in the following pair undergoes faster SN1 reaction? [1]

Answer:
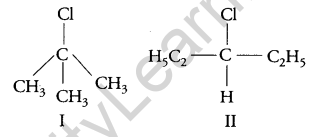
The carbocation formed when compounds I and II undergo SN1 reaction are shown below:
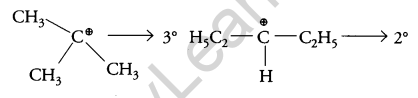
As 3° carbocation is more stable than 2° carbocation, hence compound I undergo faster SN1 reaction.
Question 8.
Write the structure of a p-Methylbenzaldehyde molecule. [1]
Answer:
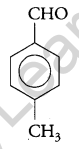
Question 9.
What is the difference between multi-molecular and macromolecular colloids? Give one example of each. [2]
Answer:
Difference between Multi-molecular and Macromolecular colloids:
| Point of Difference | Multi molecular Colloid | Macro molecular Colloid |
| Definition | When a large number of atoms or small molecules (having diameters of less than 1 nm) of a substance combine together in a dispersion medium to form aggregates having a size in the colloidal range, the colloidal solutions thus formed are called multimolecular colloid. | When substances which have very high molecular masses are dispersed in suitable dispersion medium the resulting colloidal solutions are known as macromolecular colloids. |
| Example | Gold sol, sulphur sol. | Starch, cellulose. |
Question 14.
(a) Give an example of zone refining of metals
(b) What is the role of cryolite in the metallurgy of aluminium? [2]
Answer:
(a) Zone Refining of Metals: This method is used for the production of semiconductor and other metals of very high purity like germanium, silicon, boron, gallium and indium.
(b) Role of Cryolite in Metallurgy of Aluminium: Cryolite is added to lower the melting point of mixture and to increase the conductivity of the electrolyte.
Question 17.
Account for the following: [2]
(i) The C—Cl bond length in chlorobenzene is shorter than that in CH3-Cl.
(ii) Chloroform is stored in closed dark brown bottles.
Answer:
(i) This is due to a partial double bond character to C-Cl bond (due to resonance in C6H5Cl).
(ii) Chloroform in the presence of air gets oxidized to phosgene. Phosgene is carbonyl chloride and is represented as COCl2. To prevent the formation of phosgene, chloroform is stored in dark coloured bottles. The reaction represented as
CHCl3 + \(\frac { 1 }{ 2 }\) O2 → COCl2 + HCl
Question 18.
How will you convert: [2]
(i) Propene to Propan-1-ol?
(ii) Ethanal to Propan-2-ol?
Answer:
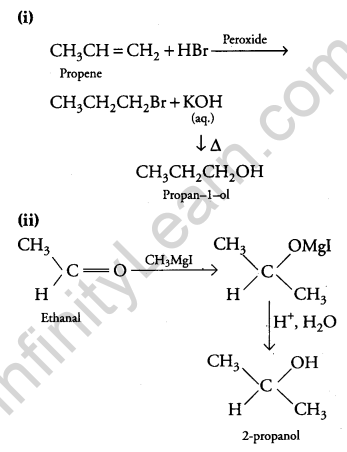
Question 23.
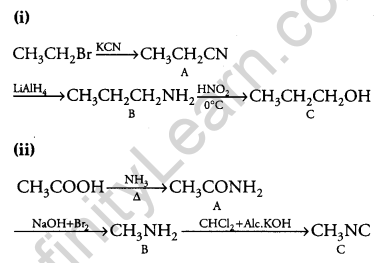
Give the structures of products A, B and C in the following reactions: [3]
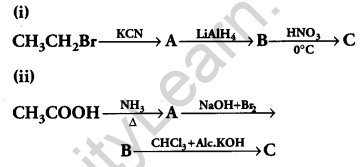
Answer:
Question 27.
Write the names and structures of the monomers of the following polymers: [3]
(i) Bakelite
(ii) Nylon-6
(iii) Polythene
Answer: