Table of Contents
Introduction to Acetic Anhydride
Acetic anhydride is a vital organic compound with a broad range of applications in various industries. Its unique formula, structure, and properties make it an essential component in many chemical reactions and processes.
In this article, we will explore the various aspects of acetic anhydride, from its molecular composition to its numerous applications.
Acetic Anhydride Formula
The chemical formula of acetic anhydride is (CH3CO)2O. It is composed of two acetyl (CH3CO) groups linked by an oxygen atom. This formula plays a crucial role in understanding its reactivity and behavior in different reactions.
Acetic Anhydride IUPAC Name
The Acetic Anhydride IUPAC Name is ethanoic anhydride. This systematic naming follows the rules of the International Union of Pure and Applied Chemistry (IUPAC) for naming organic compounds.
Acetic Anhydride Structure
The acetic anhydride structure consists of two acetyl groups joined by an oxygen atom in the center. The acetyl group (CH3CO) is composed of a methyl group (CH3) and a carbonyl group (CO). The arrangement of these atoms determines its chemical properties and functions.
The general structure of acetic anhydride is as follows:
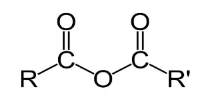
R and R’ represent alkane groups.
The structure of acetic anhydride is shown below.
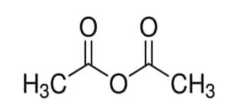
This is a molecule of acetic anhydride. When the R and R’ groups from above are substituted by methane (-CH3) groups, we get the acetic anhydride formula. This molecule is a basic example of an acid anhydride.
Acetic Anhydride Molecular Weight
The acetic anhydride molecular weight is approximately 102.09 g/mol. This value is calculated based on the atomic masses of the individual atoms in its chemical formula.
Acetic Anhydride Price
The price of acetic anhydride can vary depending on factors such as purity, quantity, and market demand. It is commonly available on the chemical market and is used in both large-scale industrial processes and smaller laboratory-scale reactions.
Physical Properties of Acetic Anhydride
- Acetic Anhydride Density
The acetic anhydride density is approximately 1.08 g/cm3. This property is essential to understanding its behavior when mixed with other substances and its solubility in different solvents.
- Acetic Anhydride Boiling Point
The boiling point of acetic anhydride is approximately 138.5°C. This property is crucial to understanding its behavior when subjected to heating during various chemical reactions.
Acetic Anhydride Uses
Acetic anhydride finds extensive use in the chemical industry, where it serves as a versatile reagent and solvent. One of its primary applications is the acetylation of various compounds, such as cellulose and morphine, to produce derivatives used in pharmaceuticals and other industries.
Acetic Acid
Acetic anhydride is closely related to acetic acid (CH3COOH), which is a weak acid commonly known as vinegar. Both compounds share similar chemical characteristics but exhibit distinct reactivity due to their different molecular structures.
Acetyl Chloride
Acetyl chloride (CH3COCl) is another organic compound related to acetic anhydride. While both compounds contain acetyl groups, acetyl chloride is a more reactive and corrosive substance, commonly used in organic synthesis.
Conclusion
In conclusion, acetic anhydride plays a crucial role in various chemical reactions and industrial processes due to its unique formula, structure, and properties. Its applications extend across several industries, making it a significant component in the world of organic chemistry.
As we delve deeper into the world of chemical compounds, understanding acetic anhydride and its diverse uses becomes essential for aspiring chemists and researchers alike.
Frequently Asked Questions on Acetic Anhydride
Is CH3COOH a type of acid anhydride?
Acetic anhydride is an organic substance with the chemical formula (CH3CO)2O, whereas acetic acid is an organic compound with the chemical formula CH3COOH.
What are the physical properties of acetic anhydride?
Acetic anhydride is a colourless liquid with a sour taste and a pungent odour. Acetic anhydride has a high density; it is slightly larger than the density of water (0.999 g/mL), making it heavier than water. The boiling point of acetic anhydride is predicted to be 139.5 degrees Celsius.
What are the applications of acid chloride?
The applications of acid chloride are mentioned below: Chemistry reagents. Pharmaceutical production. Organic compound synthesis. Peroxides are derived from organic sources. Agrochemical manufacturing. Plastics and pigments.
What is the purpose of acetic acid?
Acetic anhydride, vinyl acetate monomer, acetic esters, cellulose acetate, chloroacetic acid, plastics, dyes, pesticides, photographic chemicals, and rubber are all made from acetic acid.
What is the chemical formula for acid chloride?
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound made of a chlorine atom bonded to an acyl group.








