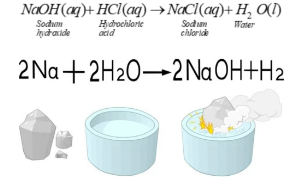Table of Contents
In this article, we will study the difference between Endothermic and Exothermic Reactions. In chemistry, there are so many reactions, and they are further divided into sub and main reactions. In Endothermic and Exothermic Reactions, energy is either released or absorbed in the form of heat, cold, sound or light.
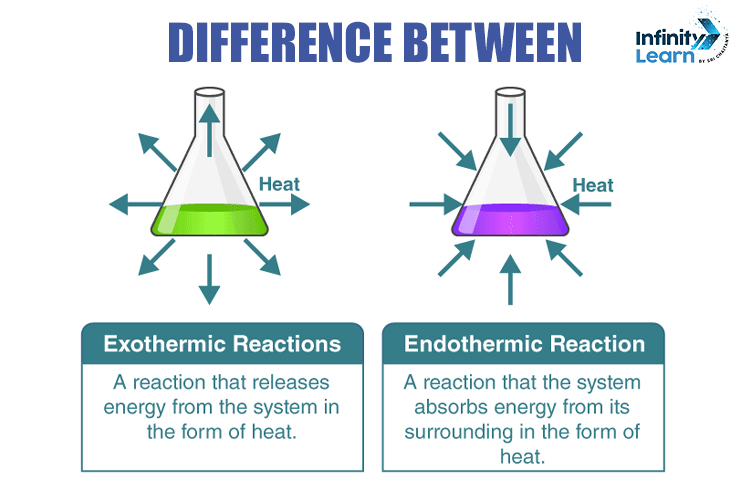
Reactions that absorb heat from the surroundings are called Endothermic reactions, whereas the reactions in which heat is released are called exothermic reactions.
Endothermic Reactions
Endothermic is made up of two words: endo means “to absorb”, and thermic means “to heat”.
Endothermic Reactions are the reactions in which the energy is absorbed by the system in the form of heat. Some examples of Endothermic reactions include photosynthesis, evaporating liquids, thermal decomposition, alkaline cracking and many more.
When the reaction is taking place, reactants are converted into the products, and as a result energy is produced. Energy is produced because bonds present in the molecules are dissociated. When the new bonds are formed, energy is released through them.
The temperature is cooler at the place where the reaction is taking place because heat is taken up from the surroundings.
In Endothermic Reactions, enthalpy increases at the end of the reaction.
Exothermic Reactions
Exothermic is made up of two words: exo means “to release”, and thermic means “heat”.
Exothermic Reactions are the opposite of Endothermic Reactions. In these reactions, energy is released in the form of heat or light. Examples: neutralisation, reactions of fuel, respiration, deposition of dry ice and many more.
When new bonds are formed, energy is released. Therefore, in exothermic reactions, enthalpy decreases.
Difference between Endothermic and Exothermic Reactions
The words endo and exo mean “to absorb” and “to release” respectively. As suggested by the names, the major difference between the Endothermic and Exothermic Reactions is that one absorbs heat while the other releases heat.
The difference between Endothermic and Exothermic Reactions is shown in the table given below
| Difference between Endothermic and Exothermic Reactions | |
| Endothermic Reactions | Exothermic Reactions |
| In Endothermic Reactions, heat is absorbed by reactants from the surroundings to form products. | In exothermic reactions, energy is released in the form of heat or light. |
| Energy is absorbed from the surroundings. | Energy is released into the surroundings. |
| Energy is absorbed in the form of heat. | Energy is released in the form of heat, electricity, light or sound. |
| In Endothermic reactions, surrounding temperature decreases. | In exothermic reactions, surrounding temperature increases. |
| The product obtained is less stable. | The product obtained is more stable. |
| Change in enthalpy is a positive value. | Change in enthalpy is a negative value. |
| The enthalpy of the product is more than the reactant. | The Enthalpy of the product is less than the reactant. |
| For example, evaporation, cooking, photosynthesis and melting of ice. | For example, the rusting of an iron, explosion and nuclear fission. |
Why is heat released or absorbed in a chemical reaction?
A chemical reaction takes place when a chemical compound changes its chemical composition. With the help of chemical bonds, molecules join together to make a chemical compound. There is a requirement of change in energy in these chemical bonds for forming or breaking of bonds respectively. And when the formation of chemical bonds takes place, energy is released in the form of heat. Energy is absorbed from the surroundings when chemical bonds are broken.
Some examples of Exothermic Reactions
It is a process in which a reaction between a substance and oxygen takes place as a result, heat and energy are released.
All combustion reactions are exothermic. We can take the example of the combustion of methane as well
![]()
The reaction between acid and base takes place to give salt and water. The reaction between Sodium Hydroxide and hydrochloric acid is a neutralisation reaction as salt and water are produced as a product
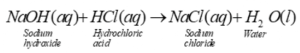
- Sodium metal in water
Enough amount of heat is given during the reaction of sodium metal and water. Sodium reacts strongly with water. H2 is produced in this exothermic reaction.
- Respiration
Respiration is a process in which carbon is taken, and oxygen is released. This is an Exothermic Reaction
Some examples of Endothermic Reactions
Plants prepare their food on their own with the help of photosynthesis. Energy is absorbed by the sun in the presence of carbon dioxide and water. As a result of which, glucose and oxygen are produced.
The reaction can be summarised as
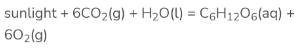
- Cooking an egg
To cook an egg, heat is absorbed from the pan.
- Melting ice cubes
Ice is added to a drink to cool it. So the ice absorbs all the heat from the water to melt. As a result, the drink becomes cool.
- Evaporation of water
In evaporation, the liquid state is changed into a gaseous state. Drying wet clothes is one example of the evaporation of water. Water droplets present inside the clothes absorb heat from the surroundings.
- Formation of carbon disulphide
The reaction between carbon and sulfur at high temperatures produces carbon disulphide
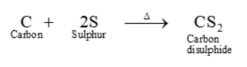
There is a change in energy in every chemical reaction. Based on this change, chemical reactions are divided into two types
- Exothermic
- Endothermic
In exothermic reactions, heat is released, which causes an increase in temperature.
In Endothermic reactions, heat is absorbed, which causes a fall in temperature.
FAQs on Difference Between Exothermic and Endothermic Reactions
Mention the reason for respiration considered to be an Exothermic Reaction
During respiration, energy is released. Carbon dioxide present in food breaks to form glucose and this glucose combines with oxygen in our cells and releases energy
Give examples of Endothermic reactions.
Photosynthesis, evaporation, melting of ice and many more.
Write any two differences between Endothermic and Exothermic Reactions.
bsorption of heat takes place during Endothermic Reactions, whereas heat is released during exothermic reactions. There is a decrease in temperature during Endothermic Reactions, and the temperature rises during an Exothermic Reaction.