Table of Contents
Butane is an organic compound with the chemical formula C4H10. It is an alkane belonging to the family of hydrocarbons, specifically a saturated hydrocarbon. Butane exists in two isomeric forms: n-butane and isobutane.
Formula of Butane: C4H10
Structure of Butane
n-Butane:
H H H H
| | | |
H – C – C – C – C – H
| | | |
H H H H
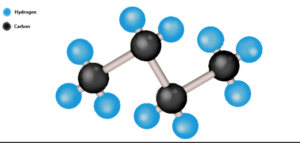
Butane
Physical Properties of Butane
- State: Butane is a colorless, odorless gas at room temperature and atmospheric pressure.
- Boiling Point: The boiling point of butane is approximately -0.5°C for n-butane and -11.7°C for isobutane.
- Density: The density of butane is about 2.48 g/L for n-butane and 2.52 g/L for isobutane at 0°C and 1 atm.
- Solubility: Butane is insoluble in water but soluble in organic solvents such as ethanol, ether, and chloroform.
Chemical Properties of Butane
- Combustibility: Butane is highly flammable and burns readily in the presence of an ignition source, producing carbon dioxide and water as combustion products.
- Halogenation: Butane can undergo halogenation reactions, such as chlorination or bromination, in the presence of halogen gases or halogenating agents.
- Isomerization: Isobutane can be isomerized to form n-butane through a process called isomerization.
- Reaction with Oxygen: Butane can react with oxygen under controlled conditions, leading to complete combustion to produce carbon dioxide and water or incomplete combustion to form carbon monoxide and water.
- Reaction with Sulfuric Acid: Butane can react with sulfuric acid to undergo sulfonation, resulting in the formation of butyl sulfonic acid derivatives.
Applications of Butane
- Fuel: Butane is commonly used as a fuel, particularly in portable stoves, lighters, and torches due to its high energy content and clean-burning properties.
- Refrigerant: Isobutane is utilized as a refrigerant in household and commercial refrigeration systems, replacing chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) due to its lower environmental impact.
- Aerosols: Butane is an essential component in aerosol products such as hairsprays, deodorants, and spray paints, serving as a propellant.
- Extraction: It is employed in extraction processes, including botanical extraction and the production of essential oils and flavorings.
- Petrochemical Industry: Butane is used as a feedstock for the production of various petrochemicals, including ethylene and propylene, which are important building blocks for plastics and synthetic fibers.
Solved examples on the Butane Formula:
Example 1: Calculate the molar mass of butane (C4H10).
Solution:
To calculate the molar mass, we need to find the sum of the atomic masses of all the atoms in the chemical formula. The atomic masses of carbon (C) and hydrogen (H) are 12.01 g/mol and 1.008 g/mol, respectively.
Molar mass of C4H10 = (4 × 12.01 g/mol) + (10 × 1.008 g/mol)
= 48.04 g/mol + 10.08 g/mol
= 58.12 g/mol
Therefore, the molar mass of butane is approximately 58.12 g/mol.
Example 2: Write the balanced chemical equation for the complete combustion of butane (C4H10) in the presence of oxygen (O2), producing carbon dioxide (CO2) and water (H2O).
Solution: The balanced chemical equation for this combustion reaction can be determined by considering the stoichiometry of the reactants and products.
C4H10 + 13O2 → 8CO2 + 10H2O
Example 3: Write the balanced chemical equation for the isomerization of isobutane (C4H10) to form n-butane (C4H10).
Solution:
The balanced chemical equation for this isomerization reaction can be represented as:
Isobutane (C4H10) ⇌ n-Butane (C4H10)
The isomerization reactions are typically reversible so, both isobutane and n-butane can interconvert under appropriate conditions.
Also read: Chemistry Formulas
Frequently Asked Questions (FAQs) on Butane Formula
1: What is the common name of butane to all?
Answer: The common name of butane, which is universally recognized, is simply “butane.” This name is used to refer to both the n-butane and isobutane isomers of the compound.
2: What are the major components of butane?
Answer: The major components of butane are the two isomers: n-butane and isobutane.
- n-Butane: Also known as normal butane, n-butane is an alkane with the chemical formula C4H10. It is a straight-chain hydrocarbon consisting of four carbon atoms bonded in a continuous chain, with ten hydrogen atoms attached. Each carbon atom is bonded to three hydrogen atoms and one neighboring carbon atom.
- Isobutane: Isobutane is an isomer of butane with the chemical formula C4H10. It is a branched-chain hydrocarbon where three carbon atoms are in a continuous chain, and the fourth carbon atom is attached as a branch. Isobutane is also known as 2-methylpropane. It has a methyl group (CH3) attached to the second carbon atom in the chain.
3: Which gas is present in butane?
Answer: There are several notable features that make butane special:
- Flammability: Butane is highly flammable. It has a low ignition energy and burns readily when exposed to a flame or spark. This property makes it valuable as a fuel for various applications, including portable stoves, lighters, and torches.
- Clean-Burning: Butane is known for its clean-burning characteristics. When it undergoes combustion, it produces carbon dioxide (CO2) and water vapor as primary combustion products. This clean-burning property makes it an environmentally friendly fuel option.
- High Energy Content: Butane has a high energy content per unit of mass. It releases a significant amount of heat when burned, which makes it an efficient and effective fuel source for various heating and energy applications.
- Versatility: Butane is a versatile compound with numerous applications. It is widely used as a fuel in portable devices and camping equipment. It is also utilized as a propellant in aerosol products such as hairsprays, deodorants, and spray paints. Additionally, it serves as a refrigerant in household and commercial refrigeration systems.
- Volatility: Butane has a relatively low boiling point and vapor pressure, which means it readily evaporates and transitions from a liquid to a gas at normal ambient temperatures. This property makes it convenient for storage and use in pressurized containers.
- Isomeric Forms: Butane exists in two isomeric forms: n-butane and isobutane. This gives it flexibility in terms of its physical and chemical properties, allowing for different applications and uses depending on the specific isomer required.
Overall, the combination of its flammability, clean-burning nature, high energy content, versatility, and isomeric forms make butane a unique and valuable compound in various industries and everyday applications.
4: What is the main source of butane?
Answer: The main source of butane is natural gas and crude oil. It is typically obtained as a by product during the refining of crude oil and natural gas processing. Butane is present in the gaseous fraction of crude oil and natural gas reserves.
During the refining process, natural gas and crude oil are separated into different fractions based on their boiling points. Butane is one of the lighter hydrocarbons and has a relatively low boiling point, which allows it to be easily separated from other components. It is typically extracted from the natural gas stream or obtained as a component of natural gas liquids (NGLs) during the refining process.
Additionally, butane can be produced through the cracking of heavier hydrocarbons. Cracking involves breaking down complex hydrocarbons into smaller molecules, and butane can be one of the resulting products.
Once extracted or produced, butane undergoes further processing, purification, and refinement to meet specific industry requirements. It is then stored and transported as a pressurized liquefied gas in cylinders, tanks, or pipelines for various applications such as fuel, propellants, and refrigeration.
5: What are the differences between butane and propane?
Answer: The key differences between butane and propane:
- Chemical Formula: Butane has the chemical formula C4H10, while propane has the formula C3H8. This means that butane has four carbon atoms, whereas propane has three.
- Boiling Points: Butane has a lower boiling point than propane. The boiling point of butane is approximately -0.5°C, while propane boils at -42°C. This difference in boiling points affects their storage and use.
- Vapor Pressure: Propane has a higher vapor pressure than butane. This means that propane can vaporize more readily at lower temperatures and pressures compared to butane. As a result, propane is commonly used in colder climates or situations where a higher vapor pressure is required.
- Energy Content: Propane has a higher energy content per unit volume compared to butane. When burned, propane releases more heat energy, making it a more efficient fuel for heating applications.
- Applications: Both butane and propane have various applications. Butane is commonly used in portable stoves, lighters, and camping equipment. Propane is frequently used for residential and commercial heating, cooking, and as a fuel for vehicles.
These are some of the key differences between butane and propane, including their chemical composition, boiling points.




