Table of Contents
Magnesium Chloride Formula
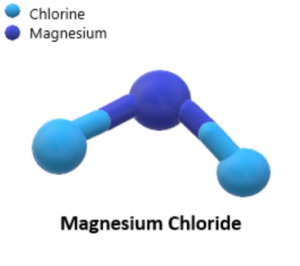
Magnesium chloride is an inorganic compound with the chemical formula MgCl2. It is composed of one magnesium ion (Mg2+) and two chloride ions (Cl–). This compound is highly soluble in water and exists as a white crystalline solid at room temperature.
Magnesium chloride is commonly obtained from natural sources such as brine wells or seawater. It is also produced through the reaction of magnesium oxide (MgO) or magnesium hydroxide (Mg(OH)2) with hydrochloric acid (HCl).
The Formula of Magnesium Chloride
The chemical formula of magnesium chloride is MgCl2. It indicates that each magnesium ion (Mg2+) is surrounded by two chloride ions (Cl-) in the compound.
Structure of Magnesium Chloride
Magnesium chloride forms an ionic crystal lattice structure. The magnesium cation (Mg2+) has a 2+ charge and is relatively small, while the chloride anion (Cl–) has a 1- charge and is larger. In the crystal lattice, the magnesium ions are surrounded by six chloride ions, forming an octahedral coordination arrangement. This arrangement contributes to the stability of the crystal lattice.
Physical Properties of Magnesium Chloride
- State: Magnesium chloride exists as a white crystalline solid at room temperature.
- Melting Point: It has a relatively high melting point of around 714 degrees Celsius, indicating its strong ionic bonds.
- Solubility: Magnesium chloride is highly soluble in water, and the resulting solution conducts electricity due to the presence of free ions.
- Hygroscopic: It is hygroscopic, meaning it readily absorbs moisture from the atmosphere.
- Deliquescent: Magnesium chloride can deliquesce, which means it can absorb enough moisture to dissolve and form a concentrated solution.
- Density: The density of solid magnesium chloride is approximately 2.32 g/cm³.
Chemical Properties of Magnesium Chloride
- Hydrates: Magnesium chloride can form hydrates, such as MgCl2·6H2O (hexahydrate) and MgCl2·2H2O (dihydrate), which have different physical properties.
- Dissociation: When dissolved in water, magnesium chloride dissociates into magnesium ions (Mg2+) and chloride ions (Cl-).
MgCl2 (s) ⇌ Mg2+ (aq) + 2Cl- (aq)
- Acid-Base Properties: Magnesium chloride is neutral and does not exhibit acidic or basic properties in aqueous solutions.
- Reactivity: It can undergo various chemical reactions, such as precipitation reactions, redox reactions, and complexation reactions.
- Dehydration: Upon heating, hydrates of magnesium chloride can lose water molecules, converting back to anhydrous magnesium chloride.
MgCl2·6H2O → MgCl2 + 6H2O
- Salt Formation: Magnesium chloride can react with other salts to form double salts, such as carnallite (KMgCl3·6H2O) and bischofite (MgCl2·6H2O).
- Corrosiveness: Magnesium chloride is corrosive to certain metals, and exposure to concentrated solutions can lead to corrosion.
Solved examples on Magnesium Chloride Formula
Example 1: Calculate the molar mass of magnesium chloride (MgCl2).
Solution:
To calculate the molar mass of magnesium chloride, we need to consider the atomic masses of magnesium (Mg) and chlorine (Cl).
The atomic mass of magnesium (Mg) = 24.31 g/mol
The atomic mass of chlorine (Cl) = 35.45 g/mol
Since there are two chlorine atoms in one molecule of magnesium chloride, we multiply the atomic mass of chlorine by 2.
Molar mass of magnesium chloride (MgCl2) = (1 × atomic mass of magnesium) + (2 × atomic mass of chlorine)
= (1 × 24.31 g/mol) + (2 × 35.45 g/mol)
= 24.31 g/mol + 70.90 g/mol
= 95.21 g/mol
Therefore, the molar mass of magnesium chloride (MgCl2) is 95.21 g/mol.
Example 2: A sample of magnesium chloride (MgCl2) contains 0.25 moles of magnesium ions. Calculate the number of chloride ions present in the sample.
Solution:
In one molecule of magnesium chloride (MgCl2), there are two chloride ions associated with one magnesium ion.
Given:
Moles of magnesium ions (Mg2+) = 0.25 moles
Since the ratio of magnesium ions to chloride ions in magnesium chloride is 1:2, the number of moles of chloride ions will be twice the moles of magnesium ions.
Moles of chloride ions (Cl–) = 2 × Moles of magnesium ions (Mg2+)
= 2 × 0.25 moles
= 0.5 moles
Therefore, the sample of magnesium chloride contains 0.5 moles of chloride ions.
Other Formulas:
| Magnesium Phosphate Formula |
| Aluminium Formula |
| Potassium Nitrate Formula |
| Phosphate Formula |
| Sodium Nitrate Formula |
Frequently Asked Questions (FAQs) on Magnesium Chloride Formula
Magnesium is important to many body systems, particularly to the muscles and nerves. Magnesium chloride (lack of natural magnesium in the body) is used to treat or prevent magnesium deficiency. Magnesium chloride can also be used for non-listed purposes in this Drug Guide.
Magnesium chloride Chloride. A compound inorganic consisting of one magnesium and two chloride ions. The compound is used as a source of magnesium ions in medicine which is important for many cellular activities. This was used as a cathartic and in alloys too.
This drug is a mineral supplement used in the blood to prevent and treat small quantities of magnesium. Some products are also used to treat symptoms of excessive stomach acid such as stomach pain, heartburn and indigestion with acids.
The hydroxide of magnesium is insoluble in mud. However, the solution is slightly simple if it is shaken in water and filtered out. This means that in the solution there are more hydroxide ions than in the original water. This is due to a breakdown of some magnesium hydroxide.
Solid magnesium chloride is an electrical nonconductor since the ions are not free to pass. But, as the ions are free on melting, it undergoes electrolysis. Magnesium chloride form neutral in the water as it forms from strong acid HCl and strong base Mg(OH)2.
Magnesium chloride is an inorganic compound with the chemical formula MgCl2. What is magnesium chloride used for?
What is magnesium chloride made of?
What are the health benefits of magnesium chloride?
Is magnesium soluble or insoluble?
Is magnesium chloride acidic or basic?
What is Magnesium Chloride Formula?








