Table of Contents
Introduction to Benzoic Acid Formula
Benzoic acid, also known as benzene carboxylic acid, is a white crystalline compound with the chemical formula C7H6O2. It is derived from benzene and contains a carboxyl group (-COOH) attached to a phenyl ring. The molecular structure of benzoic acid consists of a benzene ring with a single carbon atom bonded to both a carboxyl group and a hydrogen atom.
Formula of Benzoic Acid: C7H6O2
Structure of Benzoic Acid:
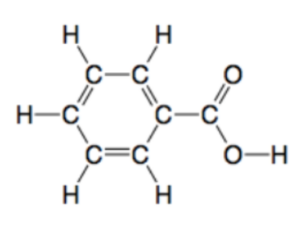
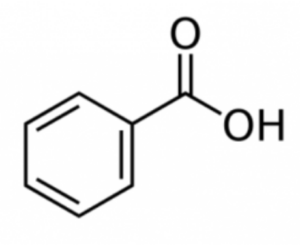
Physical Properties of Benzoic Acid
- Melting Point: Benzoic acid has a melting point of 122-123°C, making it a solid at room temperature.
- Solubility: It is sparingly soluble in cold water but more soluble in hot water, alcohol, and ether.
- Odor: Benzoic acid has a characteristic, slightly pleasant odor.
- Appearance: It appears as white crystals or powder.
Chemical Properties of Benzoic Acid
- Acidic Nature: Benzoic acid is a weak acid and can release a hydrogen ion when dissolved in water, making it slightly acidic.
- Reaction with Bases: It reacts with bases to form water-soluble salts called benzoates. For example, reacting with sodium hydroxide (NaOH) produces sodium benzoate.
C6H5COOH + NaOH → C6H5COONa + H2O - Ester Formation: Benzoic acid can undergo esterification reactions with alcohols in the presence of an acid catalyst, forming esters. This reaction is commonly used in the synthesis of various flavoring compounds.
C6H5COOH + R-OH → C6H5COOR + H2O - Decarboxylation: Upon heating, benzoic acid can undergo decarboxylation to produce benzene. This reaction is catalyzed by copper or iron and occurs at high temperatures.
C6H5COOH → C6H6 + CO2 - Oxidation: Benzoic acid can be oxidized to produce benzaldehyde or further oxidized to benzoic acid’s most common oxidation product, benzoin.
C6H5COOH → C6H5CHO + CO2
C6H5COOH → C6H5CHOHCOOH
Applications of Benzoic Acid
- Food Preservative: Benzoic acid and its salts, such as sodium benzoate, are widely used as food preservatives due to their antimicrobial properties, preventing the growth of bacteria, yeast, and molds.
- Pharmaceutical Industry: It is used in the synthesis of various pharmaceuticals, such as antifungal agents, antiseptics, and analgesics.
- Flavoring Agent: Benzoic acid and its derivatives are employed as flavoring agents in food and beverages.
- Chemical Intermediates: It serves as an important intermediate in the production of dyes, plastics, perfumes, and resins.
Solved Example on Benzoic Acid
Example 1: Calculate the molar mass of benzoic acid (C7H6O2).
Solution:
To calculate the molar mass, we need to find the sum of the atomic masses of all the atoms in the chemical formula. The atomic masses of carbon (C), hydrogen (H), and oxygen (O) are 12.01 g/mol, 1.008 g/mol, and 16.00 g/mol, respectively.
Molar mass of C7H6O2 = (7 × 12.01 g/mol) + (6 × 1.008 g/mol) + (2 × 16.00 g/mol)
= 84.07 g/mol + 6.048 g/mol + 32.00 g/mol
= 122.118 g/mol
Therefore, the molar mass of benzoic acid is approximately 122.118 g/mol.
Example 2: Reaction with Sodium Hydroxide
Write the balanced chemical equation for the reaction between benzoic acid and sodium hydroxide (NaOH) to form sodium benzoate and water.
Solution:
The balanced chemical equation for this reaction can be determined by considering the stoichiometry of the reactants and products.
C6H5COOH + NaOH → C6H5COONa + H2O
Example 3: Decarbxylation Reaction
What products are formed when benzoic acid undergoes decarboxylation?
Solution:
Decarboxylation of benzoic acid involves the removal of the carboxyl group (-COOH) from the molecule. The main product formed is benzene (C6H6), along with the release of carbon dioxide (CO2).
C6H5COOH → C6H6 + CO2
Frequently asked Question on Benzoic Acid
What is benzoic acid used for?
Benzoic acid is a preservative in food and drinks, and also in personal care products, keeping them safe from harmful microbes. It helps extend shelf life of products.
What is benzoic acid formula?
The formula of benzoic acid is C7H6O2. It consists of seven carbon, six hydrogen, and two oxygen atoms.
Is benzoic acid a base or acid?
Benzoic acid is indeed an acid. It's a weak acid that is used in a variety of applications for its antifungal and antibacterial properties.
Is it safe to drink benzoic acid?
In small amounts, benzoic acid is safe. However, consuming pure benzoic acid or high amounts can be harmful and is not advised. Always adhere to safety guidelines.
What is the formula for the structure of benzoic acid?
The formula for the structure of benzoic acid is C6H5COOH.
What are the three important reaction of benzoic acid?
The three important reactions of benzoic acid are: Esterification: Benzoic acid can undergo esterification reactions with alcohols in the presence of an acid catalyst, forming esters. Decarboxylation: Upon heating, benzoic acid can undergo decarboxylation to produce benzene. Oxidation: Benzoic acid can be oxidized to produce benzaldehyde or further oxidized to its most common oxidation product, benzoin.
What is an interesting fact about benzoic acid?
An interesting fact about benzoic acid is that it has been used as a food preservative for centuries. It was first isolated from gum benzoin, a resin obtained from certain tree species, and has been employed as a natural preservative in various foods and beverages due to its antimicrobial properties.
What is the solubility of benzoic acid formula?
The solubility of benzoic acid in water is relatively low at room temperature. It in water at 25°C is approximately 0.34 grams per 100 milliliters of water. However, it is more soluble in hot water.
Is benzoic acid harmful to use?
Benzoic acid is used in foods and medicines and is of low hazard at the concentrations used. It is generally categorized as slightly hazardous in industrial use with no special transport requirements and is biodegradable. Eating it or rubbing it in your eyes would not be advised, and workers have to be protected from daily exposure to dust which would be irritating, etc.
What are the medicinal uses of benzoic acid?
Benzoic acid aids in preventing infection produced by bacteria. Salicylic acid, a benzoic acid derivative, assists the body hut rough or discarded skin cells. Salicylic acid and benzoic acid topical is a combination medicine used to treat skin irritation and eczema, fungal infections, insect bites, or inflammation caused by burns.








