Table of Contents
Barium chloride, with the chemical formula BaCl2, is an inorganic compound composed of barium cations (Ba2+) and chloride anions (Cl–). It is a white crystalline solid that is widely used in various applications due to its distinctive physical and chemical properties.
Formula and Structure of Barium Chloride:
The formula of barium chloride, BaCl2, represents its chemical composition. It consists of one barium ion (Ba2+) and two chloride ions (Cl–). The barium ion is a divalent cation, formed by the loss of two electrons from a barium atom (Ba). The chloride ion is a monovalent anion composed of a single chlorine atom (Cl).
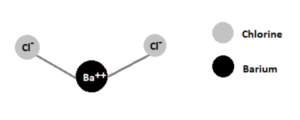
Barium chloride
The structure of barium chloride can be visualized as a crystal lattice arrangement of barium ions and chloride ions. The barium ions are positioned at the center of the lattice, surrounded by chloride ions. This ionic arrangement contributes to the overall stability of the compound.
Physical Properties of Barium Chloride:
Appearance: Barium chloride typically exists as a white crystalline solid. It is often found in powdered form or as colorless crystals.
Odor: Barium chloride is odorless.
Solubility: Barium chloride is highly soluble in water. When added to water, it readily dissolves, forming a clear solution. The solubility of barium chloride decreases with decreasing temperature.
Melting Point: The melting point of barium chloride is relatively high, around 962 degrees Celsius (1,764 degrees Fahrenheit).
Density: Barium chloride has a relatively high density of approximately 3.86 grams per cubic centimeter. The density of the compound may vary slightly depending on the temperature and pressure.
Chemical Properties of Barium Chloride:
- Hygroscopicity: Barium chloride is hygroscopic, meaning it has a tendency to absorb moisture from the surrounding environment. It can form hydrates, compounds with water molecules incorporated into their structure, when exposed to humid conditions.
- Water of Crystallization: Barium chloride can form hydrates with varying degrees of hydration. For example, the dihydrate form of barium chloride (BaCl2·2H2O) contains two water molecules per formula unit.
- Acidic Nature: Barium chloride is not acidic in itself. However, when dissolved in water, it can release barium ions, which have the potential to hydrolyze and form a slightly acidic solution.
- Barium Sulfate Precipitation: Barium chloride is commonly used as a reagent for the detection and quantification of sulfate ions (SO42-) in solutions. It reacts with sulfate ions to form insoluble barium sulfate (BaSO4), which appears as a white precipitate.
- Toxicity: Barium chloride and other barium compounds are toxic if ingested or inhaled. They can have harmful effects on the cardiovascular, nervous, and respiratory systems.
- Flame Coloration: Barium chloride is often employed in pyrotechnics and fireworks due to its ability to produce a green flame when ignited. The green color originates from the barium ions releasing energy in the form of light.
- Chemical Stability: Barium chloride is relatively stable under normal conditions. However, it can decompose at high temperatures, releasing toxic chlorine gas (Cl2).
- Reducing Agent: Barium chloride can act as a reducing agent in certain chemical reactions. For example, it can reduce chromium compounds to chromium(II) ions.
Solved examples on the formula of Barium Chloride (BaCl2):
Example 1: Calculating the Molar Mass of Barium Chloride.
To determine the molar mass of barium chloride (BaCl2), we need to sum up the atomic masses of each element in the formula.
Solution:
Atomic mass of barium (Ba) = 137.33 g/mol
Atomic mass of chlorine (Cl) = 35.45 g/mol (x 2 since there are 2 chloride ions in BaCl2)
Molar mass of BaCl2 = (137.33 g/mol) + (35.45 g/mol x 2) = 208.78 g/mol
Therefore, the molar mass of barium chloride is approximately 208.78 g/mol.
Example 2: Determining the Number of Moles in a Given Mass
Suppose we have 50 grams of barium chloride (BaCl2), and we want to calculate the number of moles present.
Solution:
Molar mass of BaCl2 = 208.78 g/mol
Number of moles = Mass / Molar mass
Number of moles = 50 g / 208.78 g/mol ≈ 0.239 moles
Therefore, there are approximately 0.239 moles of barium chloride in 50 grams.
Example 3: Stoichiometry and Reaction Calculation.
Let’s consider a balanced chemical equation representing the reaction between barium chloride (BaCl2) and sodium sulfate (Na2SO4).
Solution:
BaCl2 + Na2SO4 → BaSO4 + 2 NaCl
Suppose we have 10 moles of barium chloride (BaCl2). We can calculate the number of moles of other substances involved in the reaction.
From the balanced equation, the stoichiometry ratio between BaCl2 and BaSO4 is 1:1
Number of moles of BaSO4 = 10 moles (since the ratio is 1:1)
Similarly, the stoichiometry ratio between BaCl2 and NaCl is 1:2.
Number of moles of NaCl = 2 x 10 moles = 20 moles
Therefore, if we start with 10 moles of barium chloride, we will produce 10 moles of barium sulfate and 20 moles of sodium chloride.
Frequently asked question on Barium chloride formula
What is the problem with barium chloride?
The primary concern with barium chloride is its toxicity. Barium compounds, including barium chloride, can be harmful if ingested, inhaled, or exposed to the skin. They can have adverse effects on the cardiovascular, nervous, and respiratory systems. It is important to handle barium chloride with caution and follow appropriate safety measures when working with it.
What is the use of barium chloride formula?
The formula of barium chloride (BaCl2) is primarily used in various applications such as in the production of pigments, oil refining, metal treatment, and as a laboratory reagent. It is also utilized in diagnostic imaging tests, specifically in barium sulfate contrast studies, to visualize the gastrointestinal tract.
Is BaCl2 a base or acid?
Barium chloride (BaCl2) is neither a base nor an acid. It is an ionic compound composed of barium cations (Ba2+) and chloride anions (Cl-). In water, it dissociates into these ions and does not significantly contribute to the acidity or basicity of the solution. However, the chloride ion (Cl-) is a weak base, so in certain reactions, it may exhibit basic characteristics.
Why is BaCl2 solution always neutral in nature?
BaCl2 solution is neutral in nature because it does not produce or release significant amounts of hydrogen ions (H+) or hydroxide ions (OH-) when dissolved in water. The dissociation of BaCl2 into barium ions (Ba2+) and chloride ions (Cl-) does not contribute significantly to the acidity or basicity of the solution. Hence, the solution remains neutral.
Write the formula of Barium Chloride?
Barium is a Group 2 element, hence in its ionic form, it donates one valence electron (total two) to each chloride ion (of Group 17), creating an ionic compound. Barium forms Ba2+ and chloride means Cl− To form neutral compound they combined in 1:2 ratio. The formula for barium chloride is BaCl2.




