Magnesium oxide Formula
Magnesium oxide (MgO) is an inorganic compound consisting of magnesium and oxygen. It is commonly referred to as magnesia and occurs naturally as the mineral periclase. Magnesium oxide is a white, odorless solid with a high melting point.
Additionally, magnesium oxide is used as a supplement to provide the body with magnesium, an essential mineral that plays a crucial role in various biological processes. It is important for maintaining healthy bones, nerve function, muscle contraction, and heart rhythm.
Magnesium oxide can be produced through the heating of magnesium hydroxide or magnesium carbonate. It is also formed as a by product during the production of magnesium metal.
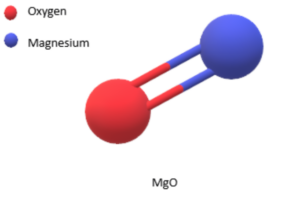
The formula of Magnesium oxide
The chemical formula of magnesium oxide is MgO. It indicates that one magnesium ion (Mg2+) is combined with one oxygen ion (O2-) in the compound.
Structure of Magnesium oxide
Magnesium oxide adopts a crystal lattice structure. The magnesium cation (Mg2+) has a 2+ charge, and the oxygen anion (O2–) has a 2- charge. In the crystal lattice, the magnesium ions are surrounded by six oxygen ions, forming an octahedral coordination arrangement. This arrangement contributes to the stability of the crystal lattice.
Physical Properties of Magnesium oxide
- State: Magnesium oxide exists as a white solid at room temperature.
- Melting Point: It has a high melting point of around 2,800 degrees Celsius, indicating its strong ionic bonds.
- Solubility: Magnesium oxide is sparingly soluble in water, meaning only a small amount dissolves. It forms a slightly alkaline solution.
- Density: The density of solid magnesium oxide is approximately 3.58 g/cm³.
- Appearance: Magnesium oxide is a fine white powder.
Chemical Properties of Magnesium oxide
- Acid-Base Properties: Magnesium oxide is a basic oxide, meaning it can react with acids to form salts and water through a neutralization reaction. For example:
MgO + 2HCl → MgCl2 + H2O
- Oxidation-Reduction Reactions: Magnesium oxide can undergo oxidation-reduction reactions, particularly when heated in the presence of oxygen. It readily reacts with oxygen to form magnesium oxide:
2Mg + O2 → 2MgO
- Reactivity with Water: Magnesium oxide does not react significantly with water at room temperature. However, it can react with water vapor at high temperatures to form magnesium hydroxide:
MgO + H2O → Mg(OH)2
- Insoluble in Organic Solvents: Magnesium oxide is insoluble in organic solvents such as ethanol or acetone.
- Refractory Properties: Due to its high melting point and resistance to heat, magnesium oxide is used as a refractory material in applications that require high-temperature stability, such as in furnace linings or crucibles.
- Antacid and Pharmaceutical Use: Magnesium oxide is commonly used as an antacid to neutralize excess stomach acid and relieve symptoms of indigestion or heartburn. It can also be used as a dietary supplement to provide magnesium ions.
Solved examples involving magnesium oxide:
Example 1: Calculating the formula mass of magnesium oxide
Solution:
To determine the formula mass of magnesium oxide, we need to consider the atomic masses of magnesium (Mg) and oxygen (O).
Magnesium (Mg) atomic mass = 24.31 g/mol
Oxygen (O) atomic mass = 16.00 g/mol
Since magnesium oxide has one atom of magnesium and one atom of oxygen, we can add their atomic masses to find the formula mass:
Formula mass of magnesium oxide = atomic mass of Mg + atomic mass of O
= 24.31 g/mol + 16.00 g/mol
= 40.31 g/mol
Therefore, the formula mass of magnesium oxide is approximately 40.31 g/mol.
Example 2: Balancing the chemical equation for the formation of magnesium oxide
Solution:
The balanced equation for the reaction between magnesium and oxygen to form magnesium oxide is:
2 Mg + O2 -> 2 MgO
This equation shows that two moles of magnesium react with one mole of oxygen to produce two moles of magnesium oxide. The balanced equation ensures that the number of atoms of each element is the same on both sides of the equation.
Example 3: Determining the percentage composition of magnesium and oxygen in magnesium oxide
Solution:
To find the percentage composition of magnesium and oxygen in magnesium oxide, we need to calculate the mass of each element relative to the total mass of the compound.
Assume we have 100 g of magnesium oxide.
The molar mass of magnesium oxide is 40.31 g/mol (as determined in Example 1).
Example 4: Calculating the mass of magnesium:
Solution:
The molar mass of magnesium is 24.31 g/mol.
The molar ratio of magnesium to magnesium oxide is 1:1.
Therefore, the mass of magnesium in 100 g of magnesium oxide is (24.31 g/mol / 40.31 g/mol) × 100 g = 60.29 g.
Example 5: Calculating the mass of oxygen:
Solution:
The molar mass of oxygen is 16.00 g/mol.
The molar ratio of oxygen to magnesium oxide is 1:1.
Therefore, the mass of oxygen in 100 g of magnesium oxide is (16.00 g/mol / 40.31 g/mol) × 100 g = 39.71 g.
Thus, in 100 g of magnesium oxide, there is approximately 60.29 g of magnesium and 39.71 g of oxygen.
To determine the percentage composition, we divide the mass of each element by the total mass of magnesium oxide and multiply by 100.
Percentage composition of magnesium = (60.29 g / 100 g) × 100% ≈ 60.29%
Percentage composition of oxygen = (39.71 g / 100 g) × 100% ≈ 39.71%
Therefore, in magnesium oxide, magnesium constitutes about 60.29% of the compound’s mass, while oxygen constitutes about 39.71%.
Frequently asked questions on Magnesium oxide
1: What is the common name of magnesium oxide?
Answer: The common name for magnesium oxide is “magnesia.”
2: What are 5 physical properties of magnesium oxide?
Answer: Here are five physical properties of magnesium oxide:
- Appearance: Magnesium oxide is a white, crystalline solid. It typically appears as a fine powder or granules.
- Density: The density of magnesium oxide varies depending on its form and manufacturing process. It typically ranges from 3.58 to 3.64 grams per cubic centimeter (g/cm³).
- Melting Point: Magnesium oxide has a high melting point. It melts at approximately 2,800 degrees Celsius (5,072 degrees Fahrenheit), making it suitable for applications that require resistance to high temperatures.
- Solubility: Magnesium oxide has a low solubility in water. It is practically insoluble, meaning it does not dissolve readily in water. However, it can react with acids to form soluble magnesium salts.
- Electrical Conductivity: Magnesium oxide is an insulator, meaning it does not conduct electricity. It has high electrical resistivity and is often used as an electrical insulating material in various applications.
These properties contribute to the diverse applications of magnesium oxide, including its use as a refractory material, antacid, and dietary supplement, among others.
3: What is magnesium oxide made up of?
Answer: Magnesium oxide (MgO) is composed of the elements magnesium (Mg) and oxygen (O). It has a chemical formula of MgO, indicating that each magnesium oxide unit contains one atom of magnesium and one atom of oxygen.
Magnesium is an alkaline earth metal, while oxygen is a nonmetal. In magnesium oxide, the magnesium atom donates two electrons to the oxygen atom, forming an ionic bond. The magnesium atom loses two electrons, becoming a positively charged ion (Mg²⁺), and the oxygen atom gains two electrons, becoming a negatively charged ion (O²⁻). The resulting ionic compound, magnesium oxide, is electrically neutral.
The chemical equation representing the formation of magnesium oxide is:
2 Mg + O2 -> 2 MgO
This equation shows that two atoms of magnesium react with one molecule of oxygen gas to produce two units of magnesium oxide.
4: What type of compound is magnesium oxide?
Answer: Magnesium oxide (MgO) is classified as an ionic compound. It is formed through the combination of magnesium (Mg), which loses two electrons to form a 2+ cation, and oxygen (O), which gains two electrons to form a 2- anion. The resulting electrostatic attraction between the oppositely charged ions creates the ionic bond in magnesium oxide. Ionic compounds consist of a lattice structure composed of positively charged ions (cations) and negatively charged ions (anions), held together by strong electrostatic forces.
5: What is the role of magnesium oxide in medicine and as a dietary supplement?
Answer: Magnesium oxide is often used in medicine and as a dietary supplement due to its role in providing magnesium, an essential mineral that plays a crucial role in various biological processes. Magnesium is necessary for maintaining healthy bones, nerve function, muscle contraction, and heart rhythm. As a result, magnesium oxide is utilized as a supplement to ensure an adequate intake of magnesium in the diet. Additionally, it is sometimes used as an antacid to alleviate symptoms of heartburn, indigestion, and upset stomach by neutralizing excess stomach acid.




