Table of Contents
In chemistry, particularly for students in Class 9, it’s crucial to grasp the difference between homogeneous and heterogeneous mixtures. This understanding is not just a part of the curriculum but also a fundamental concept that underpins many chemical and physical processes around us.
When we talk about what is a homogeneous mixture and what is a heterogeneous mixture, we delve into the very basics of material composition, a knowledge that aids in comprehending more complex scientific phenomena.
What is a Homogeneous Mixture?
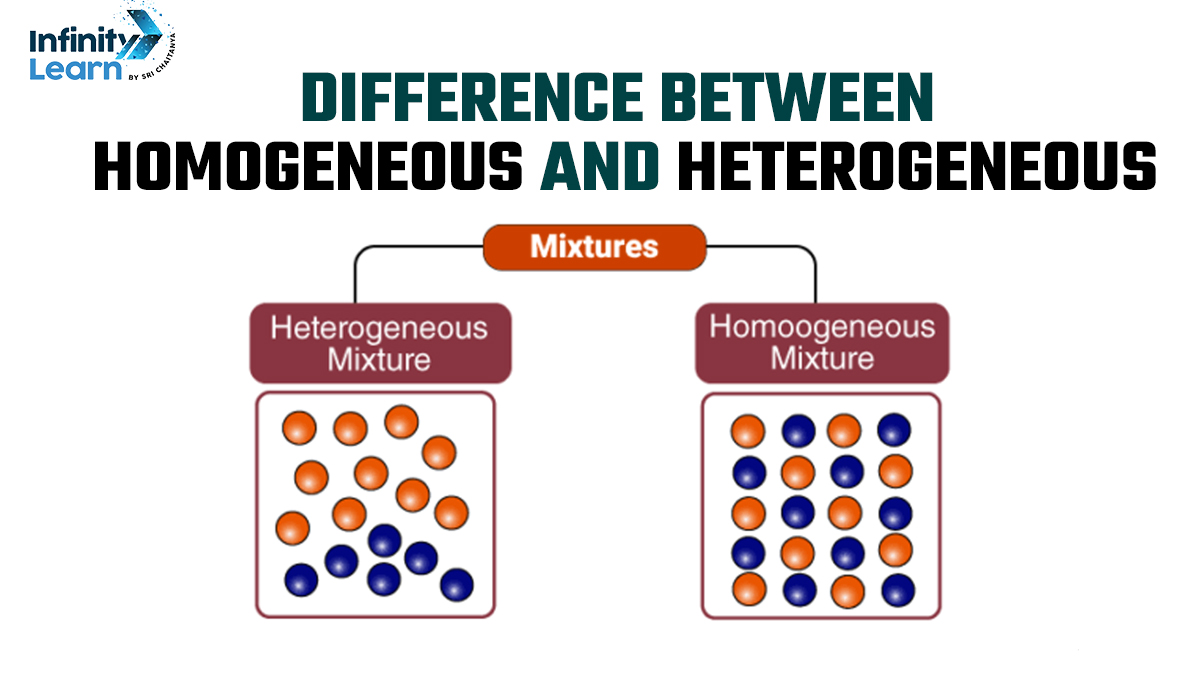
A Homogeneous Mixture is a type of mixture where the composition is uniform and consistent throughout. The components are so thoroughly mixed that they become indistinguishable from each other. An everyday example of a homogeneous mixture is salt dissolved in water.
Here, the salt disperses evenly, forming a consistent solution. Another example can be air, a mixture of various gases like nitrogen, oxygen, and trace amounts of other gases, all blended uniformly. In discussing the homogeneous and heterogeneous differences, it’s important to note that homogeneous mixtures have the same proportions of components throughout.
Examples –
- Sugar dissolved in water: You cannot see any individual sugar crystals in the solution, even when magnified.
- Saltwater: The salt is completely dissolved in the water, making the mixture appear uniform.
- Air: You cannot see the individual molecules of nitrogen, oxygen, and other gases that make up air.
What is a Heterogeneous Mixture?
In contrast, a Heterogeneous Mixture is a mixture where the components remain distinct, and their composition varies throughout the sample. For instance, a salad is a heterogeneous mixture because you can identify and separate the lettuce, tomatoes, cucumbers, and other ingredients.
Similarly, sandy water is another example where sand particles and water are visibly different components. The distinction between what is homogeneous and what is heterogeneous lies in the visibility and separability of the individual components in a heterogeneous mixture.
Examples –
- Sand and water: You can clearly see the individual sand particles in the water.
- Oil and water: The oil and water form separate layers because they are immiscible (do not mix).
- Salad: You can see different ingredients like lettuce, tomatoes, and cucumbers in the salad.
Differentiate Between Homogeneous and Heterogeneous Mixtures
| Homogeneous Mixtures | Heterogeneous Mixtures |
| Uniform composition | Non-uniform composition |
| Components are indistinguishable | Components are distinguishable and separable |
| Cannot be separated by physical methods | Can often be separated by physical methods |
| Appears as a single phase | May appear as multiple phases |
| Examples: Saltwater, air | Examples: Salad, sandy water |
| Consistent properties throughout | Variable properties in different parts |
| Solutions are a common type | Suspensions and colloids are common types |
| Light passes through without scattering | Light may scatter (Tyndall effect) |
| Doesn’t settle on standing | Components may settle on standing |
| Examples are often liquid solutions | Examples can be solid, liquid, or gaseous |
What is Homogeneous and Heterogeneous Mixture?
Understanding the differentiate between homogeneous and heterogeneous mixtures extends beyond mere academic knowledge. Here are ten reasons why this understanding is vital:
- Foundational Chemistry Knowledge: It lays the groundwork for advanced chemical study.
- Real-world Application: Many everyday products are either homogeneous or heterogeneous mixtures.
- Industrial Relevance: In industries, knowing the mixture type helps in designing processes.
- Waste Management: Differentiating mixtures is crucial in waste segregation and recycling.
- Food Industry: This knowledge is essential in food processing and preservation.
- Pharmaceuticals: Drug formulation often involves creating specific types of mixtures.
- Environmental Science: Understanding pollution often involves analyzing mixtures in the environment.
- Scientific Inquiry Skills: It fosters critical thinking and scientific inquiry.
- Material Science: Plays a role in the development of new materials and products.
- Enhances Observational Skills: Encourages students to observe and categorize materials around them.
The Tyndall Effect
The Tyndall effect is a phenomenon observed in colloidal solutions, which are mixtures containing particles dispersed in another medium but not dissolved in it. These particles are larger than individual molecules but smaller than those in a suspension. When a light beam is passed through a colloidal solution, the particles scatter the light, making the path of the light beam visible. This scattered light is what we see as the Tyndall effect.
The Tyndall effect is named after John Tyndall, who first described it in 1869. It is a useful tool for distinguishing between true solutions and colloidal solutions. True solutions do not exhibit the Tyndall effect, as the particles are too small to scatter light.
Here are some key points about the Tyndall effect
- It is caused by the scattering of light by colloidal particles.
- It is visible when a strong beam of light is passed through a colloidal solution in a darkroom.
- The scattered light is usually blue in color, due to the Rayleigh scattering of light.
- The intensity of the scattered light depends on the size and concentration of the particles in the colloidal solution.
Examples of the Tyndall effect
- The beam of light from a projector or flashlight in a smoky room
- The headlights of a car in fog
- The blue color of the sky
NCERT questions related to the Tyndall effect
- Define a colloid and distinguish it from a true solution and a suspension.
- Describe the Tyndall effect and explain its cause.
- Identify examples of the Tyndall effect in everyday life.
- Explain the importance of the Tyndall effect in various applications, such as medical diagnostics and environmental monitoring.
NCERT Questions Based on Difference Between Homogeneous and Heterogeneous Mixture
Long Answer Type Questions
- Describe the role of homogeneous mixtures in industrial chemical processes. Provide an example to illustrate your answer.
- Explain how a heterogeneous mixture can be separated using different physical methods. Use a specific example to detail the steps involved.
This question tests the student’s understanding of physical separation methods and their application to heterogeneous mixtures.
Short Answer Type Questions
- List three examples of heterogeneous mixtures commonly found in the household and identify their components.
- What is the Tyndall effect and how can it be used to distinguish between a homogeneous and a heterogeneous mixture?
- How does the concept of miscibility relate to homogeneous mixtures? Provide an example.
- Define a colloid and explain how it differs from a true solution.
- Explain why air is considered a homogeneous mixture, despite consisting of different gases.
- How would you separate a mixture of oil and water? Which category does this mixture belong to and why?
Conclusion
In conclusion, understanding the difference between homogeneous and heterogeneous mixtures is not just a curriculum necessity for Class 9 students but a fundamental aspect of comprehending the world around us.
From simple kitchen experiments to complex industrial processes, this knowledge forms the basis of many practical applications, making it a cornerstone of scientific education and inquiry.
FAQs on Difference Between Homogeneous and Heterogeneous Mixtures
What is a homogeneous mixture?
A homogeneous mixture is one that appears uniform throughout, with the same composition regardless of where you sample it. Examples include air, salt water, and brass.
What is a heterogeneous mixture?
A heterogeneous mixture exhibits a non-uniform composition, where you can see distinct components mixed together. Examples include soil, oil and water, and pizza
What is the difference between homogeneous and heterogeneous mixtures (Class 8)?
Homogeneous: Uniform appearance Mixed at molecular level Constant composition Difficult to separate Examples: Air, salt water, brass Heterogeneous: Non-uniform appearance Mixed at macroscopic level Variable composition Easy to separate Examples: Soil, oil and water, pizza








