Table of Contents
Acetate Definition: Acetate is a salt derived from acetic acid and an alkali metal or ammonium. It is a colorless, odorless, and water-soluble solid. Acetate is used to make plastics, fibers, and other industrial materials.
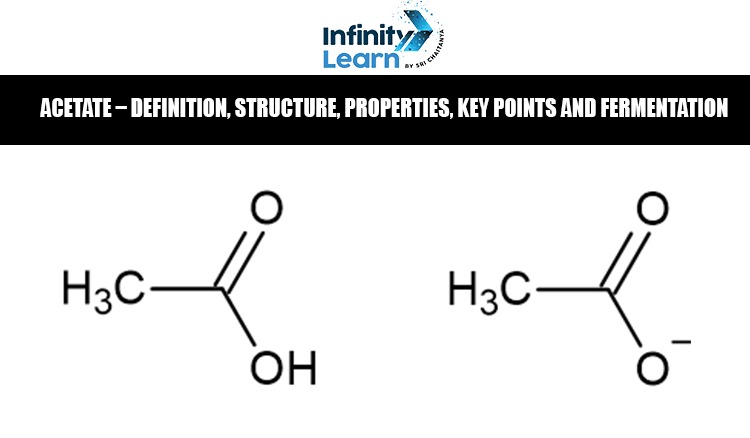
What is Acetate?
Acetate is a salt of acetic acid and is derived from cellulose. It is a colorless, odorless and tasteless liquid that is soluble in water and ethanol. It is used as a solvent, in the production of plastics and fibers, and as a pharmaceutical agent.
Acetate is a negatively charged ion (anion) derived from acetic acid and represented as CH₃COO⁻. It plays a vital role in various chemical, biological, and industrial processes. Structurally, acetate consists of a central carbon atom double-bonded to oxygen and single-bonded to a hydroxyl group, with a methyl group attached. Known for its solubility and reactivity, acetate is widely used in the production of textiles, plastics, and pharmaceuticals. In biological systems, acetate is a key metabolite involved in energy generation. During fermentation, it is produced by microorganisms as a byproduct, serving as an important intermediate in various biochemical pathways.
Acetate Structure
The acetate structure is a type of organic molecule that features a central carbon atom bonded to three oxygen atoms. The carbon atom is also bonded to one hydrogen atom, and the molecule features a chain of alternating carbon and oxygen atoms. This chain is known as an acetate group, and the molecule as a whole is known as an acetate.
Properties of Acetate (C2H3O2−)
An acetate ion is a negatively charged ion with the chemical formula C2H3O2−. It is the conjugate base of acetic acid. Acetate ions are found in a variety of weakly acidic solutions. In water, an acetate ion is surrounded by six water molecules.
Key Points of Acetate Structure
- Acetate is a type of organic compound that consists of a carbon atom double-bonded to an oxygen atom and a single-bonded to an ethyl group.
- Acetate is a versatile chemical that can be used in a variety of industries, including the production of plastics, fibers, and pharmaceuticals.
- Acetate is also an important component of vinegar, and is used as a food additive and preservative.
Fermentation of Acetate
In the absence of oxygen, acetate can be fermented to ethanol and carbon dioxide by certain bacteria, including Acetobacter and Clostridium.
Acetobacter ferments acetate to ethanol and carbon dioxide, as shown in the following equation:
2CH 3 COO- → 2CH 3 CH 2 OH + CO 2
Clostridium ferments acetate to ethanol and carbon dioxide, as shown in the following equation:
CH 3 COO- → CH 3 CH 2 OH + CO 2
Forms of Acetate
There are three main forms of acetate: acetic acid, ethylene acetate, and vinyl acetate.
- Acetic acid is a colorless liquid that is used in the production of vinegar, and it is also the main component of acetic anhydride, which is used in the production of plastics and pharmaceuticals.
- Ethylene acetate is a colorless liquid that is used as a solvent, and it is also used in the production of plastics and pharmaceuticals.
- Vinyl acetate is a colorless liquid that is used in the production of plastics and adhesives.
Chemical Reaction Used to Produce the Acetate Salt and Sodium Acetate Trihydrate
The chemical reaction used to produce the acetate salt and sodium acetate trihydrate is the reaction between acetic acid and sodium hydroxide.
Molecular Structure of the Acetate Ester, Ethyl Acetate
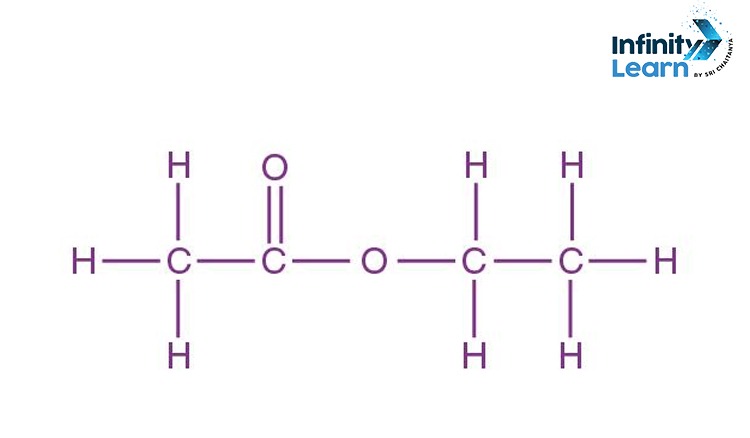
The molecular structure of ethyl acetate is shown in the figure below. The acetate ester is composed of an ethyl group (C2H5) and an acetate group (C2H4O2).
Molecular Structure of the Acetate Ester, Ethyl Acetate
Ethyl acetate is a colorless liquid that is soluble in water and has a characteristic ethereal odor. It is used as a solvent in paints, lacquers, and varnishes and as a flavoring agent in food. The molecular formula for ethyl acetate is C4H8O2.
The acetate ester functional group is characterized by the presence of a single carbon atom bonded to an oxygen atom and two carbon atoms bonded to each other. The carbon atom bonded to the oxygen atom is known as the carbonyl carbon atom. The carbon atoms bonded to each other are known as the alpha carbon atoms.
The structure of ethyl acetate can be represented by the following diagram:
The carbonyl carbon atom is bonded to the oxygen atom and the two alpha carbon atoms are bonded to each other.
Difference Between Acetate and Acetic Acid
The main difference between acetate and acetic acid is that acetate is the salt of acetic acid, while acetic acid is the acid itself. Acetate is a white, water-soluble powder, while acetic acid is a colorless, corrosive liquid. Acetic acid is also more acidic than acetate.
Acetic acid and acetate are both organic acids, but they have different structures and properties. Acetic acid is a weak acid that is made up of a carbon atom and four hydrogen atoms. Acetic acid is a colorless liquid that is soluble in water. Acetate is a salt of acetic acid that is made up of a carbon atom, a hydrogen atom, and an oxygen atom. Acetate is a colorless solid that is soluble in water.
Acetic acid is a weak acid that dissociates in water to form hydrogen ions (H+) and acetate ions (CH3COO-). The hydrogen ions react with the water to form hydronium ions (H3O+) and the acetate ions react with the water to form acetate ions (CH3COO-). The dissociation of acetic acid is shown in the following equation:
CH3COOH(aq) → H+ (aq) + CH3COO- (aq)
Acetic acid is a weak acid because it does not dissociate completely in water. Acetic acid has a pKa of 4.8. The pKa is a measure of the strength of an acid. Acetic acid is a stronger acid than water, which has a pKa of 14. Acetic acid is a weaker acid than sulfuric acid, which has a pKa of 1.
Acetate is a salt of acetic acid that is made up of a carbon atom, a hydrogen atom, and an oxygen atom. Acetate is a colorless solid that is soluble in water. Acetate is formed when acetic acid reacts with a base. The reaction of acetic acid with a base is shown in the following equation:
CH3COOH(aq) + NaOH(aq) → Na+ (aq) + CH3COO- (aq) + H2O(l)
Acetate is a weaker base than water, which has a pKa of 15.7. Acetate is a stronger base than ammonia, which has a pKa of 9.3.
Uses of an Acetate (C2H3O2−)
- The most common use of an acetate is as an acetyl group, which is a component of many molecules in biology. For example, acetylcholine is a neurotransmitter that is synthesized from acetyl CoA.
- An acetate is a salt or ester of acetic acid. Acetic acid is a colorless liquid with a pungent odor. It is the main component of vinegar.
- Acetic acid is produced industrially from ethylene.
- It is used in the production of plastics, fibers, and other materials.
- It is also used as a solvent and in the production of pharmaceuticals and other chemicals.









