Table of Contents
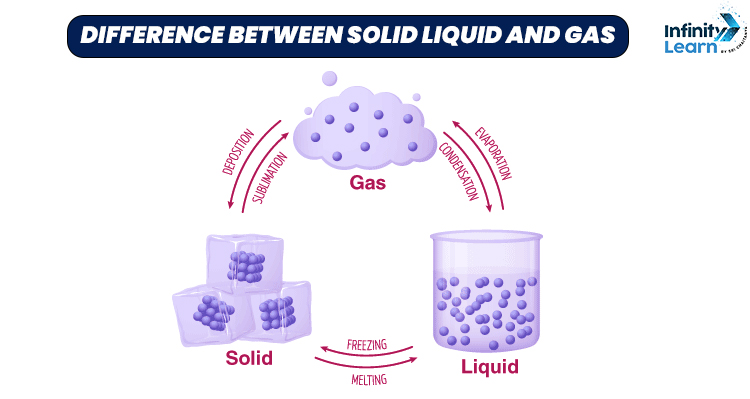
Everything around us is made up of matter. Anything that has mass and occupies space is called matter. Matter exists in three different forms, these are solid, liquid and gas. The different states of matter are due to the differences in their molecules. A solid is a form of matter which has a fixed shape and volume.
For example – wood and stone. A liquid has a fixed volume but doesn’t have a fixed shape. For example – oil and water. At the same time, gas is that form of matter which doesn’t have a fixed shape and volume. For example – nitrogen and helium.
Difference Between Solid Liquid and Gas
Below given table gives the Difference Between Solid Liquid and Gas in the tabular format.
| Property | Solid | Liquid | Gas |
| Shape | Solids have a definite shape. | Liquid does not have a fixed shape. | Gasses don’t have a fixed shape. |
| Intermolecular forces | The intermolecular forces are stronger in solids. | The intermolecular forces are stronger than gasses but weaker than solids. | The intermolecular forces are non-existent in gasses. |
| Intermolecular space | There is the least intermolecular space in solids. | Intermolecular space is more than solids but less than gasses. | Intermolecular space is the maximum in gasses. |
| Compressibility | Solids are incompressible. | Liquids cannot be compressed. | Gasses can be compressed very easily. |
| Rigidity and fluidity | Solids are very rigid and cannot flow | Liquids are less rigid and can flow very easily. | Gasses are not rigid at all and can flow very easily. |
| Diffusion | No diffusibility in solids | Slightly diffusibility. | High diffusibility |
| Kinetic energy | Particles of solids have low kinetic energy. | Molecules of liquid have intermediate kinetic energy. | Gasses have very high kinetic energy. |
| Storage | Solids can be easily stored without a container. | Liquids cannot be stored without a container. | Gasses can be stored in a vessel only. |
| Sound speed | Fastest sound speed. | Sound speed in liquids is faster than in gas but slower than in solids. | Sound speed in gasses is lowest from solids and liquids. |
| Volume | Solids have a definite volume. | Liquids have a definite volume. | Gasses don’t have a definite volume. |
| Example | Table, stone | Oil, water | Hydrogen, nitrogen |
Definition of Solids
Solid is a matter with a definite shape, distinct boundaries, fixed volumes and negligible compressibility. The intermolecular forces are stronger. Solids can maintain their shape even if an outside force is applied. They can be broken under force, but it is difficult to change their shape hence, they are rigid.
The space between the particles of solids is minimal. They are closely bound. In solids, the kinetic energy of the particles is minimal.
Definition of Liquids
Liquids have a definite volume but don’t have a fixed shape. They take up the shape of the container in which they are kept. Liquids are also called fluids, as they can flow and change shape, too, so they are not rigid. They cannot be stored without a container.
The space between the particles is intermediate in liquids. Intermolecular force is weaker than solids but stronger than gasses. Layers of particles can slip and slide over each other.
Definition of Gases
Gases don’t have fixed shapes and sizes. They can be compressed very easily as compared to solids and liquids. If we put gas in a closed container, it will expand to fill the container. The force of attraction is minimum between the particles of gasses.
The intermolecular spaces are maximum in gasses. There is no order of particles in gasses, they just move about randomly. Diffusion in gases takes place very easily.
Key Differences Between Solid, Liquid and Gas
The difference between solid liquid and gas is written below:
- A substance which has rigidity and a fixed shape is known as a solid. A water-like fluid which flows easily but has no fixed shape is known as a liquid. Gas is that state of matter which doesn’t have a fixed shape, it takes the shape of the container in which it is put.
- Solids have a fixed shape and volume, while liquids only have a fixed volume but no fixed shape. Gasses neither have fixed shape nor definite volume.
- Kinetic energy is low in solids, intermediate in liquids and highest in gasses.
- Compressibility is negligible in solids, slightly in liquids and highest in gasses.
- Solids are very rigid, liquids are less rigid, and gasses are not rigid.
- Solids cannot flow, whereas liquids and gasses can flow very easily.
- Solids can be stored without a container, whereas there is a need for a container or vessel to store liquids and gasses.
- Sound speed is highest in solids, whereas in liquids, sound speed is higher than gasses but lower than solids and minimum in gasses.
- In solids, particles are held together by strong intermolecular forces of attraction, whereas in liquids, there are low intermolecular forces of attraction due to which the particles are loosely held. In gasses, the intermolecular attraction is weak.
- For example, solids– table, wood, stone, etc. Liquids– oil, water, milk, honey, etc. Gas– helium, hydrogen, oxygen, etc.
FAQs on Difference Between Solid Liquid and Gas
Define matter?
Anything which has mass and occupies space is called matter. Everything we see around us is matter. The matter is all around us.
What are the three states of matter? Give examples
The three states of matter are solid, liquid and gas. Example - Solids- stone and wood. Liquid- milk and honey. Gas- helium and nitrogen
Define solids, liquids and gases?
A state of matter which has a definite shape and volume is solids. Liquid is a state of matter with a definite volume but no fixed shape. Gasses are a state of matter that doesn't have a fixed shape and volume
Write two properties of solids, liquids and gases?
They are not compressible They are rigid. Liquids cannot be compressed They can flow very easily Gases can be compressed very easily. They can flow very easily.









