Table of Contents
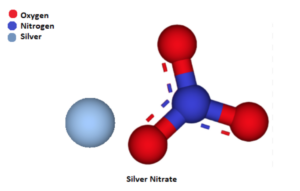
The formula for silver nitrate is AgNO₃. It represents a compound composed of one silver ion (Ag⁺) and one nitrate ion (NO₃⁻).
Formula and Structure of Silver Nitrate Formula
The molecular structure of silver nitrate consists of a silver cation (Ag⁺) bonded to the nitrate anion (NO₃⁻). The nitrate ion contains one nitrogen atom (N) bonded to three oxygen atoms (O) in a trigonal planar arrangement.
Physical Properties of Silver Nitrate Formula
- State of Matter: Silver nitrate is a white crystalline solid at room temperature.
- Solubility: It is highly soluble in water, resulting in a clear, colorless solution.
- Melting and Boiling Points: Silver nitrate has a melting point of 209.7 degrees Celsius (409.5 degrees Fahrenheit) and decomposes before reaching its boiling point.
- Odor: Silver nitrate is odorless.
Chemical Properties of Silver Nitrate Formula
- Photoreactivity: Silver nitrate is highly sensitive to light and can undergo photodecomposition when exposed to sunlight or ultraviolet (UV) radiation, resulting in the formation of metallic silver.
- 2. Precipitation Reactions: Silver nitrate is commonly used in chemical reactions to identify the presence of chloride ions (Cl⁻) or bromide ions (Br⁻). The addition of silver nitrate solution to a sample containing these ions results in the formation of white precipitates, silver chloride (AgCl) or silver bromide (AgBr), respectively.
- 3. Reactions with Reducing Agents: Silver nitrate can react with reducing agents, such as metals or organic compounds, to produce elemental silver. For example, when silver nitrate is mixed with copper metal, a redox reaction occurs, resulting in the formation of copper(II) nitrate and silver metal.
2AgNO₃ + Cu → Cu(NO₃)₂ + 2Ag
Solved Examples of Silver Nitrate Formula
Example 1: Silver Nitrate in Photography
Answer: Silver nitrate plays a crucial role in traditional black and white photography. It is used in the production of light-sensitive photographic films and papers. When exposed to light, silver nitrate undergoes a chemical reaction that results in the formation of silver particles, forming the visible image.
Also Check: Zinc Nitrate Formula
Example 2: Silver Nitrate in Medicine
Answer: Silver nitrate has been used in medical applications for its antimicrobial properties. It can be applied topically as a caustic agent to burn or cauterize tissue and to treat conditions like warts or granulation tissue. Additionally, it has been used as an ingredient in certain antimicrobial solutions and ointments.
Frequently asked question of Silver Nitrate Formula
1: Is AgNO3 acidic or basic?
Answer: Silver nitrate (AgNO₃) is considered an acidic compound. When dissolved in water, it dissociates to release Ag⁺ ions, which do not have any significant acidic or basic properties. However, the nitrate (NO₃⁻) ions can react with water to form nitric acid (HNO₃), contributing to the acidic nature of the solution.
2: What is the other name for AgNO3?
Answer: Another name for AgNO₃ is silver nitrate is argentum nitricum.
3: What happens when AgNO3 is added to NaCl?
Answer: When silver nitrate (AgNO₃) is added to sodium chloride (NaCl) solution, a chemical reaction occurs. The silver cation (Ag⁺) from silver nitrate reacts with the chloride anion (Cl⁻) from sodium chloride to form a white precipitate of silver chloride (AgCl). The balanced chemical equation for this reaction is
AgNO₃ + NaCl → AgCl + NaNO₃
In this reaction, the silver chloride is insoluble in water and appears as a white solid precipitate. The sodium nitrate (NaNO₃) remains in the solution. The formation of the white precipitate of silver chloride is a commonly used test for the presence of chloride ions in solution.
4: What are 3 facts about silver nitrate?
Answer: There are 3 facts about silver nitrate
- Antimicrobial Properties: Silver nitrate has long been recognized for its antimicrobial properties. It is used in various medical applications, such as wound care, eye drops for newborns to prevent infection, and in the production of antimicrobial creams and ointments.
- Photographic Uses: Silver nitrate is essential in traditional black and white photography. It is used in the production of light-sensitive films and papers. When exposed to light, silver nitrate undergoes a chemical reaction that forms silver particles, producing the visible image.
- Chemical Reactions: Silver nitrate is involved in several chemical reactions. It is commonly used in analytical chemistry to detect the presence of chloride or bromide ions, forming precipitates of silver chloride (AgCl) or silver bromide (AgBr). Additionally, silver nitrate can react with reducing agents to produce elemental silver.
5: What are the uses of silver nitrate?
Answer: Silver nitrate is widely used in many organic synthesis reactions in several ways. For example, for the deprotection and oxidation reactions. The Ag+ ion reversibly binds alkenes, and selectively adsorbing silver nitrate can be used to isolate alkene mixtures. The resulting adduct can be decomposed (in order to release the free alkene) with ammonia. Silver nitrate has been, in the past, used for silver staining (a process that employs silver or silver compounds to selectively change the appearance of a specific object). This compound is also used in medicine owing to its antiseptic qualities.







