Table of Contents
Amino Acids Formula
Amino acids are organic compounds that contain an amino group (-NH2), a carboxyl group (-COOH), and a side chain group (R group) attached to a central carbon atom. These compounds serve as the fundamental building blocks of proteins. Here is a general representation of an amino acid structure:
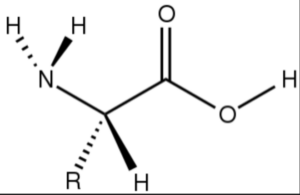
The specific structure and properties of an amino acid depend on the nature of its side chain (R group). There are 20 standard amino acids commonly found in proteins, each with a unique side chain. However, for brevity, I will provide the formula and structure of the simplest amino acid, glycine.
Glycine:
Formula: C2H5NO2
Physical properties of amino acids
- State: Amino acids can exist as solids or liquids at room temperature, depending on their specific properties.
- Solubility: Most amino acids are soluble in water due to the presence of hydrophilic functional groups (-NH2 and -COOH). However, some amino acids with nonpolar side chains are relatively hydrophobic and have limited solubility in water.
Chemical properties of amino acids
- Acidic and basic properties: Amino acids possess both acidic (carboxyl group) and basic (amino group) properties. The amino group can accept a proton, acting as a base, while the carboxyl group can donate a proton, acting as an acid.
- Zwitterionic nature: In a neutral pH environment, amino acids exist as dipolar ions called zwitterions. The amino group is positively charged, and the carboxyl group is negatively charged, resulting in overall neutrality.
- Peptide bond formation: Amino acids can undergo condensation reactions, forming peptide bonds between the carboxyl group of one amino acid and the amino group of another. This process leads to the formation of proteins.
It’s important to note that the physical and chemical properties of amino acids can vary depending on the specific amino acid and its side chain. The 20 standard amino acids exhibit a wide range of properties, which contribute to the diverse structures and functions of proteins in living organisms.
List of 20 Amino Acids
- Alanine – Ala – A
- Arginine – Arg – R
- Asparagine – Asn – N
- Aspartic acid – Asp – D
- Cysteine – Cys – C
- Glutamine – Gln – Q
- Glutamic acid – Glu – E
- Glycine – Gly – G
- Histidine – His – H
- Isoleucine – Ile – I
- Leucine – Leu – L
- Lysine – Lys – K
- Methionine – Met – M
- Phenylalanine – Phe – F
- Proline – Pro – P
- Serine – ser – S
- Threonine – Thr – T
- Tryptophan – Trp – W
- Tyrosine – Tyr – Y
- Valine – Val – V
Common amino acids, including their formula, structure, chemical properties, and uses
Glycine
- Formula of glycine: C2H5NO2
- Structure of glycine: H2N-CH2-COOH
- Chemical properties of glycine: Glycine is the simplest amino acid and is nonpolar, achiral, and hydrophilic.
- Uses of glycine: It plays a crucial role in protein synthesis and is used in various pharmaceutical and cosmetic applications.
Alanine
- Formula of Alanine: C3H7NO2
- Structure of Alanine: CH3-CH(NH2)-COOH
- Chemical properties of Alanine: Alanine is a nonpolar amino acid and is hydrophobic.
- Uses of Alanine: It is essential for the metabolism of glucose and is used in the production of pharmaceuticals, food additives, and flavorings.
Valine
- Formula of valine: C5H11NO2
- Structure of valine: (CH3)2-CHCH(NH2)-COOH
- Chemical properties of valine: Valine is a branched-chain amino acid (BCAA) and is hydrophobic.
- Uses of valine: It is involved in muscle metabolism, tissue repair, and the production of energy. Valine supplements are used by athletes and bodybuilders.
Leucine
- Formula of leucine: C6H13NO2
- Structure of leucine: (CH3)2-CHCH2CH(NH2)-COOH
- Chemical properties of leucine: Leucine is a branched-chain amino acid (BCAA) and is hydrophobic.
- Uses of leucine: It plays a crucial role in protein synthesis, muscle building, and energy production. Leucine supplements are used in sports nutrition.
Phenylalanine
- Formula of Phenylalanine: C9H11NO2
- Structure of Phenylalanine: C6H5-CH2-CH(NH2)-COOH
- Chemical properties of Phenylalanine: Phenylalanine is an aromatic amino acid and is hydrophobic.
- Uses of Phenylalanine: It is used as a nutritional supplement and is a precursor for the synthesis of neurotransmitters like dopamine and norepinephrine.
Solved examples on Amino Acid
Example 1: Alanine (C3H7NO2)
Solution:
Alanine is a nonpolar amino acid with a hydrophobic side chain.
Example 2: Glutamine (C5H10N2O3)
Solution:
Glutamine is a polar amino acid with a hydrophilic side chain.
Frequently Asked Question on Amino Acid
Which amino acids appear most frequently?
In terms of the 20 standard amino acids, some amino acids appear more frequently than others in proteins. The relative frequency of amino acids can vary depending on the specific protein and organism.
However, here are four amino acids that are generally considered to be among the most frequently occurring in proteins:
Leucine (Leu): Leucine is an essential amino acid and is often found in high abundance in proteins. It has hydrophobic properties and plays a crucial role in protein structure and function.
Glycine (Gly): Glycine is the smallest amino acid and is also commonly found in proteins. It is unique in that it has a hydrogen atom as its side chain, making it highly flexible. Glycine is often found in regions where flexibility and mobility are important, such as protein hinges and turns.
Alanine (Ala): Alanine is a nonpolar amino acid and is frequently present in proteins. It is involved in protein synthesis and is known for its role in stabilizing protein structures.
Serine (Ser): Serine is a polar amino acid and is relatively common in proteins. It plays various roles, including serving as a phosphorylation site for signaling pathways and contributing to the catalytic activity of enzymes.
The frequency of amino acids can vary depending on the specific protein, organism, and the functional requirements of the protein. Additionally, other amino acids such as lysine, glutamine, and valine are also commonly found in proteins, albeit with varying frequencies.
What are 3 uncommon amino acids?
Among the 20 standard amino acids, there are a few that are considered relatively uncommon compared to others due to their occurrence in fewer proteins or specialized roles. Here are three examples of relatively uncommon amino acids: Selenocysteine (Sec): Selenocysteine is an amino acid that contains selenium instead of sulfur in its side chain. It is incorporated into certain proteins via a unique mechanism. Selenocysteine is involved in antioxidant defense and has important roles in enzymes and proteins associated with selenium metabolism. Pyrrolysine (Pyl): Pyrrolysine is an unusual amino acid that contains a pyrroline ring. It is found in some methanogenic archaea and is involved in the enzymatic production of methane. Pyrrolysine is incorporated into proteins via a specialized translational machinery. Norleucine (Nle): Norleucine, also known as norvaline, is an analog of leucine. It has a shorter carbon chain in its side group compared to leucine. Norleucine can be incorporated into proteins, primarily as a result of errors during translation or post-translational modifications. It is considered uncommon but can be found in some proteins.
What are the 3 most important amino acids?
he three most important amino acids are: - Glycine (Gly): Glycine is the simplest amino acid and plays a crucial role in the synthesis of proteins and other important molecules in the body. It is a nonpolar amino acid and serves as a building block for collagen, enzymes, and neurotransmitters. -Glutamine (Gln): Glutamine is an essential amino acid involved in many metabolic processes. It is particularly important for the health and function of the digestive system, immune system, and muscle tissue. Glutamine is often used as a supplement by athletes and individuals with certain medical conditions. -Lysine (Lys): Lysine is an essential amino acid that the body cannot produce on its own and must be obtained from the diet. It is involved in protein synthesis, collagen formation, and the production of enzymes and hormones. Lysine is important for proper growth and development, and it also has antiviral properties.
What is the rarest amino acid in proteins?
The rarest naturally occurring amino acid in proteins is selenocysteine (Sec). Selenocysteine is an unusual amino acid that contains selenium instead of sulfur, which is typically found in cysteine. It is coded by a unique codon, known as the UGA codon, which is usually a stop codon in the genetic code. However, in specific contexts and with the help of specific RNA structures and enzymes, the UGA codon can be recoded to incorporate selenocysteine during protein synthesis. Due to its rarity and specialized incorporation mechanism, selenocysteine is found in only a small number of proteins involved in specific functions such as antioxidant defense and selenium metabolism.
What are essential amino acids?
Essential amino acids are amino acids that cannot be synthesized by the human body and must be obtained through diet. There are nine essential amino acids: histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine. These amino acids are crucial for protein synthesis and various physiological functions.
What is the difference between essential and nonessential amino acids?
Nonessential amino acids are those that the human body can synthesize on its own, so it is not necessary to obtain them directly from the diet. In contrast, essential amino acids cannot be synthesized in adequate amounts by the body and must be obtained from external sources such as food.
What is the role of amino acids in protein synthesis?
Amino acids are the building blocks of proteins. During protein synthesis, amino acids are joined together through peptide bonds to form polypeptide chains. This process involves the translation of genetic information stored in DNA into the sequence of amino acids, which determines the structure and function of the resulting protein.
What are branched-chain amino acids (BCAAs)?
Branched-chain amino acids (BCAAs) are a subgroup of essential amino acids that have a branched side chain. The three BCAAs are leucine, isoleucine, and valine. They play a crucial role in muscle metabolism, energy production, and protein synthesis. BCAA supplements are popular among athletes and bodybuilders for their potential benefits in muscle recovery and performance enhancement.
What are the functions of amino acids in the body?
Amino acids have diverse functions in the body, including: Protein synthesis: Amino acids are the building blocks of proteins, which are essential for growth, repair, and maintenance of body tissues. Enzyme production: Amino acids are involved in the synthesis of enzymes, which catalyze biochemical reactions in the body. Neurotransmitter synthesis: Certain amino acids act as precursors for neurotransmitters, which are chemical messengers involved in signaling between nerve cells. Energy production: Amino acids can be used as an energy source when glucose levels are low. Immune system support: Amino acids are involved in immune system function and play a role in antibody production and immune cell activity.








